For patients with hormone receptor-positive breast cancer, an important means of preventing recurrence is anti-estrogen therapy. Among them, tamoxifen and aromatase inhibitors are commonly used drugs. How are they used, how effective are they and who are they suitable for? A recent article published in The Lancet Oncology[1] examines this issue in greater depth. Xiaobian takes everyone to take a look.
Where does estrogen come from?
Estrogen is one of the most important hormones for women, and can be synthesized in the body and can also be ingested from outside the body. Estrogens include estradiol, estrone, and estriol, of which estradiol has the highest physiological activity.
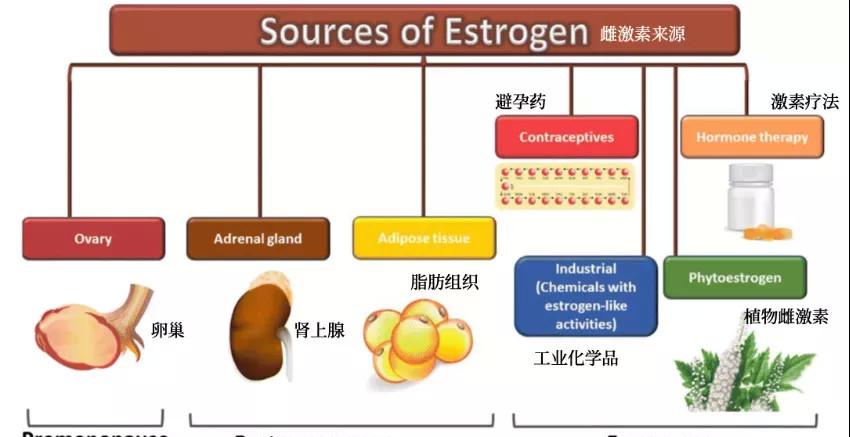
Figure 1. Estrogen Sources[2]
Endogenous estrogen
In premenopausal women, the ovaries are the main source of estrogen (estradiol);
In postmenopausal women, the ovaries no longer produce estrogen, but estrogen (estrogen) can still be produced in the body. How is estrone produced? An androgen secreted by the adrenal glands is catalyzed by aromatase. Adipose tissue is a great source of aromatase, and expression and activity increase with age. In addition to postmenopausal women, prepubertal children and men also produce estrogen through this route.
Exogenous estrogen
Exogenous compounds with estrogen-like activity include contraceptives, hormone replacement therapy, phytoestrogens, and industrial chemicals.
What are the anti-estrogen therapies?
Depending on the source of estrogen, the drugs used and the mechanism of action are different.
Tamoxifen: Can compete with estrogen to bind to estrogen receptors, blocking the way estrogen works. In estrogen-positive, surgical breast cancer patients, tamoxifen after surgery reduces the risk of dying of breast cancer by about one-third within 15 years;
Aromatase inhibitors: Since aromatase promotes estrogen synthesis, aromatase inhibitors can block this process. For postmenopausal women, it is even more effective than tamoxifen, reducing the risk of death by another 30%.
For premenopausal women, aromatase inhibitors are ineffective because in addition to the pathway of aromatase conversion, the ovaries secrete large amounts of estrogen. In this group of patients, aromatase inhibitors should be used to simultaneously inhibit ovarian function.
Ovarian suppression therapy can also be used in conjunction with tamoxifen, and there is evidence that this combination therapy significantly prolongs disease-free survival in patients with premenopausal breast cancer3. So for premenopausal hormone-positive breast cancer patients, is tamoxifen combined with ovarian suppression chosen, or is it possible to choose aromatase inhibitors combined with ovarian suppression?
Premenopausal women: aromatase inhibitors are better
The researchers from the United Kingdom answered the question by analyzing data from four large randomized controlled trials involving more than 7,000 women with early-stage breast cancer in all countries around the world.
Each patient received an ovarian suppression treatment, such as drugs such as gosereline or tamoxifen, or surgical resection. These patients are treated with tamoxifen or an aromatase inhibitor (anastrozole, exemetam, or letrozole) for three or five years. The median follow-up was eight years.
Of the more than 7,000 patients, 888 (12.6 percent) of the women had breast cancer recurring, and 418 died, 54 of them from causes unrelated to breast cancer.
The recurrence rate of breast cancer in women receiving aromatamolxifen was significantly reduced compared to women receiving tamoxifen, especially in the first five years, the recurrence rate in the tamoxifen group was 10.1%, the recurrence rate in the aromatase inhibitor group was 6.9%, and the risk of recurrence was reduced by one-third compared to the tamoxifen group (Figure 2A). The distal transfer rate is also relatively low in the aromatase inhibitor group (Figure 2B).
There was not much difference in mortality between the two groups A (Figures 2C&D). However, the survival benefits of aromatase inhibitors may not become apparent until after a longer follow-up period, and more research is needed.
Figure 2. Recurrence rates (A), metastase rates (B), mortality rates (C&D) in the tamoxifen group (red) and aromatase inhibitor groups (blue)
Side effects are controllable
One of the side effects of aromatase inhibitors is an increased risk of osteoporosis, which can lead to fractures. In this analysis, women in the aromatase inhibitor group had a slightly higher rate of fractures during follow-up (6.4% vs 5.1%) compared to the tamoxifen group. But the overall probability of fracture is low, and this can be mitigated by using bone-strengthening drugs such as bisphosphonate.
Tamoxifen increases the risk of endometrial abnormalities, including uterine polyps and endometrial cancer. In this analysis, the five-year incidence of endometrial cancer in the tamoxifen group was higher (0.3%) compared to the aromatase inhibitor group (0.2%), although it was still generally rare.
summary
In women with premenopausal hormone-positive early-stage breast cancer who receive ovarian suppression therapy, the use of aromatase inhibitors reduces the risk of breast cancer recurrence compared to tamoxifen.
The finding has the potential to change the lives of thousands of women, especially young women, who are particularly at high risk of breast cancer recurrence. Most breast cancers are hormone-sensitive and treated with hormone therapy, and while there are some possible side effects, aromatase inhibitors can increase the chances of these women remain disease-free and allow them to return to a normal life.
bibliography:
1.Bradley, R. et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. The Lancet Oncology 0, (2022).
2.Wehbe, Z., Nasser, S. A., El-Yazbi, A., Nasreddine, S. & Eid, A. H. Estrogen and Bisphenol A in Hypertension. Curr Hypertens Rep 22, 23 (2020).
3.Kim, H.-A. et al. Adding Ovarian Suppression to Tamoxifen for Premenopausal Breast Cancer: A Randomized Phase III Trial. J Clin Oncol 38, 434–443 (2020).