▎藥明康德内容團隊編輯
5月份,根據PDUFA日程安排,FDA有可能在一個月内對3款新藥做出審評決定,詳見下表。
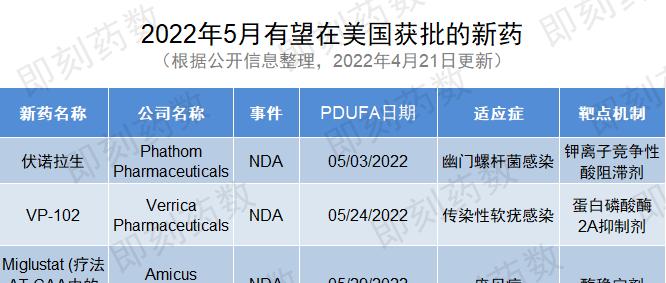
▲5月有望在美國獲批的新藥(藥明康德内容團隊制圖,點選可見大圖)
1. 藥物名稱:伏諾拉生
公司:Phathom Pharmaceuticals
适應症:幽門螺杆菌感染
伏諾拉生(vonoprazan)是一款新型鉀離子競争性酸阻滞劑(P-CAB)。該藥物由Phathom Pharmaceuticals從武田(Takeda)引進。Phathom于2021年9月送出了兩項新藥申請(NDA),尋求準許該藥物與阿莫西林和克拉黴素聯合使用,以及僅與阿莫西林聯合,用于治療成人幽門螺杆菌感染。積極的3期臨床結果顯示,在所有患者(包括克拉黴素耐藥型幽門螺杆菌感染的一部分患者)中,根除率優于基于蘭索拉唑(lansoprazole)的标準療法。伏諾拉生已獲得優先審評資格,PDUFA目标行動日期為今年5月3日。伏諾拉生已在日本、中國和拉丁美洲的某些國家獲得準許用于治療酸(如胃酸)相關疾病。如果在美國獲得準許,該公司預計最早在2022年下半年推出兩種基于伏諾拉生的治療方案,為幽門螺杆菌感染提供新的治療選擇。
最近,Phathom還向FDA送出了一份NDA,使用伏諾拉生治療糜爛性食管炎。糜爛性食管炎是一種胃食管反流病的主要類型,僅在美國就影響約2000萬人,存在着大量未滿足的需求。按照目前的公司披露的時間表,伏諾拉生用于治療糜爛性食管炎預計将在2023年上半年獲批上市。
2. 藥物名稱:VP-102
公司:Verrica Pharmaceuticals
适應症:傳染性軟疣感染
Verrica Pharmaceuticals(Verrica)的VP-102是一種藥械組合,包含蛋白磷酸酶2A抑制劑斑蝥素(0.7%溶液),通過一次性塗抹器局部給藥。Verrica正在尋求将該療法用于治療傳染性軟疣感染。軟疣是一種具有高度傳染性的常見皮膚病,在美國約有600萬人受到影響,目前沒有FDA準許的治療方法,是皮膚病中最大的未滿足需求之一。
3期臨床研究顯示,該産品在減少病變和完全清除感染方面顯示出統計學意義上的顯著療效。FDA于2021年12月接收了VP-102的NDA,并将PDUFA目标行動日期設定為今年5月24日。如果在美國獲得準許,VP-102有潛力成為軟疣的标準治療方法。
3. 藥物名稱:miglustat(療法AT-GAA中的組分)
公司:Amicus Therapeutics
适應症:龐貝病
Amicus Therapeutics(Amicus)的組合療法AT-GAA(cipaglucosidase alfa + miglustat)是治療龐貝病的酶替代療法(ERT)。Miglustat是一種口服酶穩定劑,旨在增強cipaglucosidase alfa的活性。龐貝病(糖原累積病II型)是因α-1,4-葡萄糖苷酶遺傳缺陷導緻,進而引發糖原堆積在溶酶體和胞質中,造成心肌、骨骼肌等一系列髒器損害的罕見病,發病率約為1/4萬~1/5萬。
FDA已經接收了miglustat的NDA和cipaglucosidase alfa的生物制品許可申請(BLA)。3期臨床結果顯示,AT-GAA相比标準治療方式alglucosidase alfa,在治療遲發性龐貝病患者(包括已經過ERT治療但治療需求尚未得到滿足的患者)是獲得具有臨床意義的改善效果。FDA已将miglustat的NDA的PDUFA日期設定為5月29日,cipaglucosidase alfa的BLA的PDUFA日期設定為2022年7月29日。如果獲得準許,該療法可能成為治療龐貝病的新治療标準。Amicus還在尋求歐盟準許該療法,并計劃在其他國家進一步送出監管申請。
Expected US New Drug Approvals in May 2022
In May, the FDA’s schedule remains busy, with a potential of three drugs to be approved within the month.
(By WuXi AppTec content team. Click the image to view at full size)
1. Drug: Vonoprazan
Company: Phathom Pharmaceuticals
Indication(s):Helicobacter pyloriinfection
Vonoprazan is an oral small molecule potassium-competitive acid blocker inlicensed by Phathom from Takeda. Phathom submitted two NDAs in September 2021, seeking approval of the drug in combination with amoxicillin and clarithromycin, as well as in combination with amoxicillin only, for the treatment ofHelicobacter pyloriinfection in adults.The FDA accepted the NDA filings based on positive phase III data showing superior eradication rates versus the standard-of-care lansoprazole-based triple therapy among all patients including in a subset of patients with clarithromycin-resistant strains ofH pylori, with a 6-month priority review and a PDUFA target action date of May 3, 2022.The drug has already gained approval in Japan and certain countries in Asia and Latin America for acid-related diseases. If approved in the US, the company anticipates launching the two vonoprazan-based regimens as early as H2 2022, to offer new treatment options to address the decliningH pylorieradication rates. Recently, Phathom also submitted an NDA to the FDA for the use of vonoprazan as a treatment for healing and maintenance of erosive esophagitis, a major type of gastroesophageal reflux disease affecting approximately 20 million people in the US, with significant unmet need. With the current schedule, the company expects to launch vonoprazan for erosive esophagitis in H1 2023.
2. Drug: VP-102 (cantharidin0.7% topical solution; Ycanth)
Company: Verrica Pharmaceuticals
Indication(s):Molluscum contagiosuminfection
Verrica’s VP-102 is a drug-device combination comprising the protein phosphatase 2A inhibitor cantharidin (0.7% solution), delivered topically via a single-use applicator, seeking FDA approval for the treatment ofMolluscum contagiosuminfection (molluscum).The product has shown statistically significant efficacy on reduction of lesion and complete clearance compared with vehicle in phase III studies.The FDA accepted the NDA filing of VP-102 in December 2021 and assigned a PDUFA goal date of May 24, 2022. If approved in the US, VP-102 would be marketed under the conditionally accepted trade name Ycanth. It has the potential to become the standard treatment for molluscum, a common, highly contagious skin disease that affects an estimated six million people in the US with no FDA-approved treatment and one of the largest unmet needs in medical dermatology.
3. Drug: miglustat (a component of AT-GAA)
Company: Amicus Therapeutics
Indication(s): Pompe disease
The third therapeutic awaiting FDA’s decision in May is Amicus’ miglustat, to be co-administered with cipaglucosidase alfa as a two-component therapy, AT-GAA, for the treatment of Pompe disease. Miglustat is an orally administered enzyme stabilizer, designed to enhance the activity of the synthetic enzyme replacement therapy (ERT) cipaglucosidase alfa.The FDA has accepted the NDA for miglustat and a BLA for cipaglucosidase alfa based on phase III results of AT-GAA, which showed clinically meaningful improvement with AT-GAA over the standard-of-care alglucosidase alfa, for the treatment of late onset Pompe disease, including ERT-experienced patients with high unmet need.The agency has set a PDUFA action date of May 29, 2022 for the NDA and July 29, 2022 for the BLA. If approved, the therapy could become the new standard of care for the treatment of Pompe disease. Amicus is also seeking for approval of the therapy in the EU and plans further regulatory submissions in other countries.
參考資料:
[1] Phathom Pharmaceuticals Submits Two NDAs to U.S. FDA forVonoprazan-based Treatment Regimens for the Treatment of H. pylori Infection.Retrieved April 25, 2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-submits-two-ndas-us-fda-vonoprazan-based
[2] Phathom Pharmaceuticals Reports Third Quarter 2021 Results andProvides Key Clinical, Regulatory, and Business Updates. Retrieved April 25,2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-reports-third-quarter-2021-results-and
[3] Phathom Pharmaceuticals Overview – March 2022. Retrieved April 22,2022https://investors.phathompharma.com/static-files/5ab51510-23ac-4ce8-8df6-fd0333e74334
[4] Phathom Pharmaceuticals Submits Vonoprazan NDA to FDA for theTreatment of Erosive Esophagitis. Retrieved April 25, 2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-submits-vonoprazan-nda-fda-treatment
[5] Cantharidin, a potent and selective PP2A inhibitor, induces anoxidative stress-independent growth inhibition of pancreatic cancer cellsthrough G2/M cell-cycle arrest and apoptosis. Retrieved April 25, 2022 from,https://pubmed.ncbi.nlm.nih.gov/20331621/#:~:text=Cantharidin%20is%20an%20active%20constituent,%2C%20and%20cell%2Dfate%20determination.
[6] Verrica Company Presentation – April7, 2022. Retrieved April 25, 2022 from, https://cdn.verrica.com/wp-content/uploads/2020/08/08094649/Verrica-Corporate-Presentation_April_22.pdf
[7] Verrica Pharmaceuticals Reports Fourth Quarter and Full-Year 2021Financial Results. Retrieved April 25, 2022 from,https://verrica.com/press_release/verrica-pharmaceuticals-reports-fourth-quarter-and-full-year-2021-financial-results/
[8] Enhancing Delivery of AcidAlpha-Glucosidase (GAA) to Skeletal Muscle in Pompe Disease (PD): KeyChallenges and Attributes of AT-GAA. Retrieved April 25,2022 from, https://mdaconference.org/node/1102
[9] Corporate Overview: At the Forefront of Therapies for Rare Diseases (April 2022). Retrieved April 25, 2022 from,https://ir.amicusrx.com/static-files/a0bab53e-ed7d-4af8-9bc5-f08c5714a72a
[10] Dermavant Announces FDA Acceptance for Filing of New DrugApplication (NDA) for Tapinarof Cream for the Treatment of Adults with PlaquePsoriasis. Retrieved April 25, 2022 from,https://www.dermavant.com/dermavant-announces-fda-acceptance-for-filing-of-new-drug-application-nda-for-tapinarof-cream/
[11] Dermavant Showcases New Long-Term Durability and Tolerability Datafrom Phase 3 PSOARING 3 Trial of Tapinarof Cream for Adults with PlaquePsoriasis at the 2022 AAD Annual Meeting. Retrieved April 25, 2022 from,https://www.dermavant.com/dermavant-showcases-new-data-from-phase-3-psoaring-at-the-2022-aad-annual-meeting/