▎ WuXi AppTec content team editor
In May, according to the PDUFA schedule, the FDA may make a review decision on 3 new drugs within one month, as detailed in the table below.
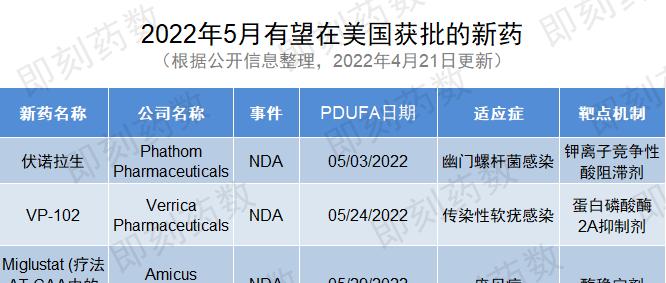
▲New drugs expected to be approved in the United States in May (WuXi AppTec content team mapping, click to see larger image)
1. Drug name: Vonorasine
Company: Phathom Pharmaceuticals
Indications: Helicobacter pylori infection
Vonoprazan is a novel potassium ion competitive acid blocker (P-CAB). The drug was introduced by Phathom Pharmaceuticals from Takeda. Phathom filed two New Drug Applications (NDAs) in September 2021 seeking approval for the drug in combination with amoxicillin and clarithromycin, and only with amoxicillin for the treatment of Helicobacter pylori infection in adults. Positive phase 3 clinical results show that in all patients, including a subset of clarithromycin-resistant Helicobacter pylori infection, eradication rates are superior to standard therapies based on lansoprazole. Vonorason has been qualified for priority review and the PDUFA target action date is May 3 this year. Vonorasan has been approved in Japan, China, and certain countries in Latin America for the treatment of acid-related diseases such as stomach acid. If approved in the U.S., the company expects to launch two vonorasin-based treatment options as early as the second half of 2022, providing new treatment options for Helicobacter pylori infection.
Recently, Phathom also submitted an NDA to the FDA for the treatment of erosive esophagitis using vonorasine. Erosive esophagitis is a major type of gastroesophageal reflux disease, affecting about 20 million people in the United States alone, with a large number of unmet needs. According to the current company-disclosed timeline, Vonorasine for the treatment of erosive esophagitis is expected to be approved for marketing in the first half of 2023.
2. Name of the drug: VP-102
Company: Verrica Pharmaceuticals
Indications: Molluscum contagiosum infection
Verrica Pharmaceuticals (Verrica)'s VP-102 is a combination of medicinal devices that contains the protein phosphatase 2A inhibitor cantharidin (0.7% solution) administered topically through a disposable applicator. Verrica is seeking to use the therapy to treat molluscum infections. Molluscum is a highly contagious common skin disease that affects about 6 million people in the United States and is currently not an FDA-approved treatment and is one of the largest unmet needs in skin diseases.
The Phase 3 clinical study showed that the product showed statistically significant efficacy in reducing lesions and completely clearing infections. The FDA received the NDA for VP-102 in December 2021 and set the PDUFA target action date for May 24 this year. If approved in the United States, VP-102 has the potential to become the standard treatment for molluscum.
3. Drug name: miglustat (component of therapy AT-GAA)
Company: Amicus Therapeutics
Indications: Pompeii disease
Amicus Therapeutics' combination therapy AT-GAA (cipaglucosidase alfa + miglustat) is an enzyme replacement therapy (ERT) for the treatment of Pompeii disease. Miglustat is an oral enzyme stabilizer designed to enhance the activity of cilaglucosidase alfa. Pompe disease (glycogen accumulation disease type II) is a rare disease caused by genetic defects in α-1,4-glucosidase, which in turn causes glycogen to accumulate in lysosomes and cytoplasm, causing a series of organ damage such as myocardium and skeletal muscle, with an incidence of about 1/40,000 to 1/50,000.
The FDA has received NDA and CIPAGLUSidase alfa bioproduct licensing applications (BLA) from Miglustat. Phase 3 clinical results showed that AT-GAA was clinically significant in the treatment of patients with delayed Pompe disease (including those who had already undergone ERT treatment but whose treatment needs had not been met) compared with the standard treatment modality, alglucosidase alfa. The FDA has set the PDUFA date for Miglustat's NDA to May 29 and the PDUFA date for cipaglucosidase alfa's BLA to July 29, 2022. If approved, the therapy could become the new treatment standard for Pompeii disease. Amicus is also seeking EU approval for the therapy and plans to file further regulatory applications in other countries.
Expected US New Drug Approvals in May 2022
In May, the FDA’s schedule remains busy, with a potential of three drugs to be approved within the month.
(By WuXi AppTec content team. Click the image to view at full size)
1. Drug: Vonoprazan
Company: Phathom Pharmaceuticals
Indication(s):Helicobacter pyloriinfection
Vonoprazan is an oral small molecule potassium-competitive acid blocker inlicensed by Phathom from Takeda. Phathom submitted two NDAs in September 2021, seeking approval of the drug in combination with amoxicillin and clarithromycin, as well as in combination with amoxicillin only, for the treatment ofHelicobacter pyloriinfection in adults. The FDA accepted the NDA filings based on positive phase III data showing superior eradication rates versus the standard-of-care lansoprazole-based triple therapy among all patients including in a subset of patients with clarithromycin-resistant strains ofH pylori, with a 6-month priority review and a PDUFA target action date of May 3, 2022.The drug has already gained approval in Japan and certain countries in Asia and Latin America for acid-related diseases. If approved in the US, the company anticipates launching the two vonoprazan-based regimens as early as H2 2022, to offer new treatment options to address the decliningH pylorieradication rates. Recently, Phathom also submitted an NDA to the FDA for the use of vonoprazan as a treatment for healing and maintenance of erosive esophagitis, a major type of gastroesophageal reflux disease affecting approximately 20 million people in the US, with significant unmet need. With the current schedule, the company expects to launch vonoprazan for erosive esophagitis in H1 2023.
2. Drug: VP-102 (cantharidin0.7% topical solution; Ycanth)
Company: Verrica Pharmaceuticals
Indication(s):Molluscum contagiosuminfection
Verrica’s VP-102 is a drug-device combination comprising the protein phosphatase 2A inhibitor cantharidin (0.7% solution), delivered topically via a single-use applicator, seeking FDA approval for the treatment ofMolluscum contagiosuminfection (molluscum). The product has shown statistically significant efficacy on reduction of lesion and complete clearance compared with vehicle in phase III studies. The FDA accepted the NDA filing of VP-102 in December 2021 and assigned a PDUFA goal date of May 24, 2022. If approved in the US, VP-102 would be marketed under the conditionally accepted trade name Ycanth. It has the potential to become the standard treatment for molluscum, a common, highly contagious skin disease that affects an estimated six million people in the US with no FDA-approved treatment and one of the largest unmet needs in medical dermatology.
3. Drug: miglustat (a component of AT-GAA)
Company: Amicus Therapeutics
Indication(s): Pompe disease
The third therapeutic awaiting FDA’s decision in May is Amicus’ miglustat, to be co-administered with cipaglucosidase alfa as a two-component therapy, AT-GAA, for the treatment of Pompe disease. Miglustat is an orally administered enzyme stabilizer, designed to enhance the activity of the synthetic enzyme replacement therapy (ERT) cipaglucosidase alfa. The FDA has accepted the NDA for miglustat and a BLA for cipaglucosidase alfa based on phase III results of AT-GAA, which showed clinically meaningful improvement with AT-GAA over the standard-of-care alglucosidase alfa, for the treatment of late onset Pompe disease, including ERT-experienced patients with high unmet need. The agency has set a PDUFA action date of May 29, 2022 for the NDA and July 29, 2022 for the BLA. If approved, the therapy could become the new standard of care for the treatment of Pompe disease. Amicus is also seeking for approval of the therapy in the EU and plans further regulatory submissions in other countries.
Resources:
[1] Phathom Pharmaceuticals Submits Two NDAs to U.S. FDA forVonoprazan-based Treatment Regimens for the Treatment of H. pylori Infection.Retrieved April 25, 2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-submits-two-ndas-us-fda-vonoprazan-based
[2] Phathom Pharmaceuticals Reports Third Quarter 2021 Results andProvides Key Clinical, Regulatory, and Business Updates. Retrieved April 25,2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-reports-third-quarter-2021-results-and
[3] Phathom Pharmaceuticals Overview – March 2022. Retrieved April 22,2022https://investors.phathompharma.com/static-files/5ab51510-23ac-4ce8-8df6-fd0333e74334
[4] Phathom Pharmaceuticals Submits Vonoprazan NDA to FDA for theTreatment of Erosive Esophagitis. Retrieved April 25, 2022 from,https://investors.phathompharma.com/news-releases/news-release-details/phathom-pharmaceuticals-submits-vonoprazan-nda-fda-treatment
[5] Cantharidin, a potent and selective PP2A inhibitor, induces anoxidative stress-independent growth inhibition of pancreatic cancer cellsthrough G2/M cell-cycle arrest and apoptosis. Retrieved April 25, 2022 from,https://pubmed.ncbi.nlm.nih.gov/20331621/#:~:text=Cantharidin%20is%20an%20active%20constituent,%2C%20and%20cell%2Dfate%20determination.
[6] Verrica Company Presentation – April7, 2022. Retrieved April 25, 2022 from, https://cdn.verrica.com/wp-content/uploads/2020/08/08094649/Verrica-Corporate-Presentation_April_22.pdf
[7] Verrica Pharmaceuticals Reports Fourth Quarter and Full-Year 2021Financial Results. Retrieved April 25, 2022 from,https://verrica.com/press_release/verrica-pharmaceuticals-reports-fourth-quarter-and-full-year-2021-financial-results/
[8] Enhancing Delivery of AcidAlpha-Glucosidase (GAA) to Skeletal Muscle in Pompe Disease (PD): KeyChallenges and Attributes of AT-GAA. Retrieved April 25,2022 from, https://mdaconference.org/node/1102
[9] Corporate Overview: At the Forefront of Therapies for Rare Diseases (April 2022). Retrieved April 25, 2022 from,https://ir.amicusrx.com/static-files/a0bab53e-ed7d-4af8-9bc5-f08c5714a72a
[10] Dermavant Announces FDA Acceptance for Filing of New DrugApplication (NDA) for Tapinarof Cream for the Treatment of Adults with PlaquePsoriasis. Retrieved April 25, 2022 from,https://www.dermavant.com/dermavant-announces-fda-acceptance-for-filing-of-new-drug-application-nda-for-tapinarof-cream/
[11] Dermavant Showcases New Long-Term Durability and Tolerability Datafrom Phase 3 PSOARING 3 Trial of Tapinarof Cream for Adults with PlaquePsoriasis at the 2022 AAD Annual Meeting. Retrieved April 25, 2022 from,https://www.dermavant.com/dermavant-showcases-new-data-from-phase-3-psoaring-at-the-2022-aad-annual-meeting/