▎ WuXi AppTec content team editor
Liver cancer is the third leading cause of cancer-related death globally, with the most common type being hepatocellular carcinoma (HCC). For early-stage hepatocellular carcinoma, surgical resection is the standard of care. However, as many as 70% of patients will still relapse postoperatively, and no perioperative intervention has shown a survival benefit.
Recently, Lancet-Gastroenterology and Hepatology recently published the results of a Phase 2 clinical trial from the Icahn School of Medicine at Mount Sinai team. Neoadjuvant immunotherapy cemiplimab (PD-1 inhibitor) has been shown to induce tumor cell death in a large area (≥50%) in nearly 1/3 of patients.
The paper notes that this is "the largest clinical trial reported to date of monotherapy for hepatocellular carcinoma PD-1 inhibitors." "The pathological response observed in the study supports the design of larger trials to further determine the optimal duration of treatment and clarify clinical benefits."
Screenshot source: The Lancet Gastroenterology & Hepatology
Previous studies have shown that neoadjuvant immunotherapy can induce pathological remission in a variety of tumor types and may reduce the risk of postoperative recurrence of hepatocellular carcinoma. Neoadjuvant immunotherapy can not only kill tumors, but also hopefully, through a long-lasting immune response, kill tiny cancer cells that are difficult to completely eliminate surgically, and these tiny cancer cells may lead to future cancer recurrence or metastasis. Therefore, this study aims to evaluate the clinical activity of cemiplimab neoadjuvant therapy in patients with resectable hepatocellular carcinoma.
It was a single-arm, open-label Phase 2 trial that included 21 patients with resectable hepatocellular carcinoma (stages Ib, II, and IIIb) who received neoadjuvant therapy (350 mg ivy with two cycles every 3 weeks), followed by surgical resection followed by postoperative cemiplimab adjuvant therapy (350 mg IV for 8 cycles every 3 weeks).
The primary endpoint of the study was significant tumor necrosis (defined as resection of the tumor > 70% necrosis). Secondary endpoints included delay in surgery, overall response rate, changes in CD8+ T cell density, and adverse events. Tumor necrosis and response rates were analyzed in all patients receiving at least one dose of cemiplimab and surgical resection; safety and other endpoints were analyzed in populations intended for treatment. The patient underwent a pre-treatment biopsy and received a blood collection throughout the treatment.
All enrolled patients received neoadjuvant therapy with cemiplimab, and 20 patients were successfully resected. Among them, 4 (20%) patients observed significant tumor necrosis after neoadjuvant immunotherapy, including 3 (15%) with complete necrosis; 7 patients (35%) with tumor necrosis rates ≥ 50%. Based on imaging results, 3 (15%) patients achieved partial remission (tumor volume reduction ≥30%), and the disease remained stable in all other patients.
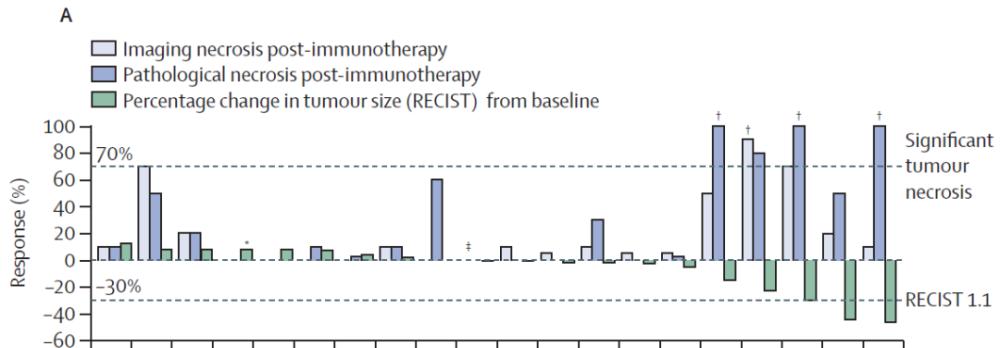
Remission (imaging) and tumor necrosis (imaging and pathology) of 20 surgical patients. (Image source: References[1])
The RESULTS OF THE MRI assessment of tumor necrosis are closely related to the results of the pathological assessment, but the results of the necrosis assessment (whether pathological or imaging) are only moderately correlated with the tumor remission results, and this correlation is not significant. This difference suggests that each indicator has its own limitations in quantifying tumor volume reduction.
Immunohistochemical analysis showed that patients with a necrosis rate of ≥ 50% tended to have more immune cells before treatment, and the immune infiltration density and tumor-infiltrating lymphocytes increased further after treatment, including CD8+ T cell infiltration. In other words, patients whose immune system is already fighting cancer before treatment tend to have more response to immunotherapy, and the immune system is further activated by immunotherapy.
The safety of treatment is acceptable. None of the patients had surgery canceled due to adverse events. A major predictable potential obstacle to neoadjuvant immunotherapy is the delay in radical surgery due to the risk of toxicity, and only one patient in this trial developed pneumonia, resulting in a 2-week delay in surgery.
20 (95%) patients experienced treatment-related adverse events of any level during neoadjuvant therapy. The most common adverse events of any level were elevated aspartate aminotransferase (4 patients), elevated creatine phosphokinase (3 patients), constipation (3 patients), and fatigue (3 patients).
Seven patients had grade 3 adverse events, including elevated creatine phosphokinase (2 cases) and hypoalbuminemia (1 case).
No level 4 or 5 events were observed.
Image credit: 123RF
"We think it's better for patients to receive immunotherapy before surgery because people are healthier before metastasis, their immune systems are in better shape, and they can better fight cancer." Combined with the results of neoadjuvant immunotherapy trials for many other types of tumors, this study supports the need to evaluate perioperative immunotherapy strategies to reduce the risk of recurrence. Study corresponding author Professor Thomas Marron, Icahn School of Medicine, Mount Sinai, said, "Future larger trials will help determine the utility, safety and survival benefits of neoadjuvant immunotherapy." ”
Concurrent review articles also point out that future studies should consider the most appropriate combination of drugs for neoadjuvant immunotherapy for liver cancer, the optimal duration of treatment, and subsequent postoperative treatment based on risk factor stratification. The correlation between early indicators of disease activity (e.g., tumor necrosis) and a persistently reduced risk of recurrence remains to be established.