"Artificial prostheses and internal fixations for the treatment of bone and joint trauma and diseases should have good antimicrobial properties in addition to the need for sufficient strength and stiffness. Some internal fixation products are best absorbed on their own after the injury has healed, eliminating the pain and expense of secondary removal surgery. It is hoped that the new materials and internal plants developed through the combination of medicine and industry in recent years can meet the above needs and be put into production and application as soon as possible to benefit patients. Academician Dai Yinrong, founder of Senfeng Medical, said so.
Orthopedic implant consumables are a market with a compound growth rate of up to 15.8%. Among them, orthopedic trauma products have always occupied the largest share of the mainland orthopedic implant consumables market. However, as Academician Dai Yinrong said, in fact, the antibacterial properties of orthopedic implant consumables have become a particularly important part of the research and development process of orthopedic implant consumables in addition to strength and stiffness.
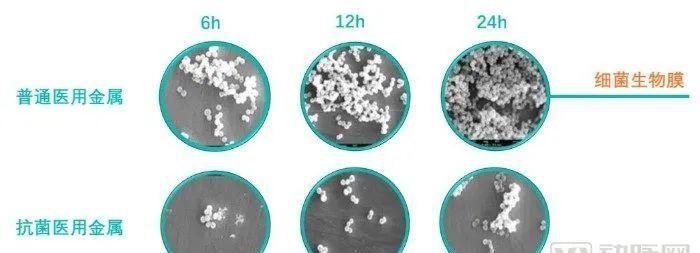
Related studies have shown that the two main causes of joint prosthesis replacement failure are aseptic loosening and infection. Approximately 18% of implant failures are due to aseptic loosening and 20% of failures are attributed to infection. Infection of the implant site caused by bacteria will cause a solid build-up on the surface of the implant. These solids not only provide an adhesion surface for bacteria to attach and proliferate. It will also further form a protective extracellular substance, the biofilm, making it difficult to remove bacteria on the attachment surface.
Therefore, the ideal implant should promote bone formation and prevent bacterial adhesion, thereby reducing the patient's bacterial infection rate.
In recent years, with the continuous development of the industry, the continuous innovation of surface coating technology, 3D printing technology, and new implant materials has become an important factor driving the development of the orthopedic implant consumables market. However, on the one hand, the high-end core materials for the research and development of raw materials for orthopedic implantable devices in China are still mainly imported and the foundation is weak. On the other hand, orthopedic implant consumables need to be implanted in the human body, and the biocompatibility, mechanical compatibility, corrosion resistance, wear resistance and other properties of product materials are required to naturally only increase and not decrease.
Therefore, to make a breakthrough in this technology, it is necessary to accumulate technology and experience.
R&D of innovative medical metal materials,
Break through the orthopedic implant "stuck neck" puzzle
In August 2019, Professor Yang Ke, a well-known expert in the field of medical metal materials in mainland China, and Academician Dai Yinrong, a well-known orthopedic clinical expert, jointly founded Suzhou Senfeng Medical Device Co., Ltd. (hereinafter referred to as "Senfeng Medical"), which is committed to the development of innovative medical metal materials and the development of high-end implantable medical devices with independent intellectual property rights based on orthopedic implants.
"Technically, antibacterial titanium alloys or antibacterial metal materials are a short-term breakthrough for us. We attach great importance to the research and development of degradable metal materials, and our technical route is mainly these two - antibacterial and degradable. Yu Yachuan, director of commercial development at Senfeng Medical, told Arterial Network.
Despite the management of the surgical procedure, the use of antimicrobial drugs, and the placement of antibacterial coatings on the surface of orthopedic implants can reduce the postoperative infection rate to some extent. However, there are still relevant studies that show that titanium coatings such as plasma spraying have good mechanical properties and biocompatibility, but it does not have bone induction ability. Due to biological inertness, the implant cannot be bone-bound to living bone tissue, which may result in aseptic loosening.
Orthopedic implants still need to find new breakthroughs to solve the pain points of the current industry.
Senfeng Medical adopts the results of the national 973 project (researched by Yang Ke's team) ontology antibacterial metal and biodegradable magnesium alloy technology, and makes full use of the performance advantages of the two innovative materials to develop original orthopedic implant products. While reflecting the advantages of materials, it meets customer needs and solves the problems that need to be solved in the clinic.
These two new materials have good biocompatibility and are superior to ordinary titanium alloys and antibacterial coating orthopedic products in terms of mechanical properties, antibacterial properties and biosafety.
Among them, the body antibacterial metal medical implant material has the biological functions of promoting osteogenesis, promoting vascularization, strong broad-spectrum antibacterial, preventing bacterial infection, etc., and has a wide range of application prospects in the medical field. At the same time, the innovative materials and innovative technologies used in the project have obtained national invention patents, and the metal materials include the body antibacterial stainless steel, the body antibacterial titanium alloy, and the body antibacterial cobalt-based alloy, etc., which are rich in antibacterial metals and can meet the medical needs in an all-round way.
Degradable magnesium alloy medical implant materials do not contain toxic elements such as rare earths, which can degrade and disappear after implantation in patients, and at the same time produce an alkaline environment and induce tissue growth. Its mechanical properties and biological properties are better than that of degradable polymer materials, and it does not have mechanical properties limitations to meet the needs of the applied products. Not only that, Senfeng Medical's patented surface treatment technology can also control the degradation rate of implant materials in patients. Effectively avoid the economic, physical and psychological secondary damage caused to patients by factors such as infection and implant removal.
Degradable magnesium alloy degradation rate of medical implant materials
Antibacterial metal and degradable magnesium alloy grasping,
Continuous layout of multiple areas
Antimicrobial orthopedic metal implants: international originality and wide application
Senfeng Medical's antibacterial orthopedic trauma implantation devices include bone needles, bone plates, bone graft screws and intramedullary nails, which belong to the third class of medical devices.
The trauma implantation device made of antibacterial titanium alloy not only retains the material and design characteristics of similar products of ordinary titanium alloy, but also increases the biological function of antibacterial (infection prevention) and promotes osteogenesis, which is the world's first, and its clinical significance has been fully recognized by the majority of orthopedic clinicians.
At present, Senfeng Medical has developed a complete range of antibacterial titanium alloy trauma products, and has obtained biological test data and antibacterial performance animal test data. Four new material trauma products– bone needles, bone plates, bone screws and intramedullary nails, will receive CE certification by May 2023.
Senfeng Medical currently has a product line
Degradable magnesium alloy implants: stable and controlled degradation
Its degradable magnesium alloy interface screw has certain advantages in mechanical properties and degradation performance, which is better than non-degradable similar products and polymer degradable products that degrade acid accumulation. At the same time, the team effectively controlled the degradation rate of the product through surface treatment technology to match the clinical healing rate and avoid non-uniform degradation of the product.
The degradable magnesium alloy interface screw developed by Senfeng Medical is a widely used implant product in the field of sports medicine, which can be used for knee anterior cruciate ligament fixation, rotator cuff injuries and other ligament injuries.
In the field of sports medicine, Senfeng Medical has achieved comprehensive coverage of its product line. Its products include degradable magnesium alloy interface screws, anterior cruciate ligament reconstruction surgical tools, body antibacterial titanium alloy bone needles, meniscus sutures, snare threads, etc.
At the same time, Senfeng Medical also targeted the vascular suture market. Senfeng Medical believes that vascular sutures are widely used in the fields of cardiology and vascular surgery, but the current market is monopolized by import manufacturers, which contains many opportunities. On the other hand, with the company's own advantages in material innovation, it can break into the market of a new generation of new material vascular suture products. At present, in order to lay the groundwork for its own new material suture products and collect market data, Senfeng Medical has developed similar alternative products for current imported products.
Yu Yachuan told Arterial Network: "For doctors, an implant that can significantly reduce the rate of infection is very attractive. This is also the reason why we will continue to deploy in the field of antimicrobials in the short term. Degradable materials are the next areas we want to lay out, because degradable materials can create additional effects at the patient's implant site, including antibacterial, local environmental pH, osteogenesis promotion, and inhibition of bone dissolution.
The development of these two technologies is particularly meaningful for the current orthopedic market. First, implant infections are a current industry and clinical challenge, and our materials and technologies are just the right ones to deliver solutions that include anti-inflammatory and antimicrobial responses. Secondly, the incidence of osteoporosis and osteoarthritis in China is very high, and the bone-building effect of our materials can provide an added solution. Finally, our technique can also inhibit bone dissolution, which is of great clinical significance. In the future, we will also lay out the company's product pipeline around these areas. ”
In the future, Senfeng Medical will also rely on its advantages in new material implanted devices to further provide solutions in the fields of energy devices (RF, ultrasound, power planing), smart devices, and customized surgical tools.
Metal biomaterials experts and orthopedic clinical experts work together to drive product innovation
Orthopedic implant consumables industry is a multidisciplinary interdisciplinary industry, the industry involves clinical medicine, materials science, surface technology, machinery manufacturing and other multidisciplinary knowledge and practice, orthopedic implant consumables industry will inevitably need to take new technologies, new materials, new processes as the driving force to promote the development of the industry.
The founding team of Senfeng Medical brings together the top biomedical material experts, orthopedic clinical experts and orthopedic implant product design team with rich experience.
Founder Yang Ke is a fellow of the International Federation of Biomaterials and Engineering and serves as a second-level research institute at the China Institute of Science and Metal. Yang Ke put forward the innovative idea of biological functionalization of medical metal materials for the first time in the world, and engaged in the research and development of new medical metal materials such as antibacterial metals, degradable metals, biological functional metals, and nuclear magneto-compatible metals. He has published more than 500 articles, authorized more than 100 invention patents, and realized multiple biomedical functions such as antibacterial, bone growth, vascularization, inhibition of smooth muscle hyperplasia, anticoagulation, anti-tumor, and anti-urethral stones of medical metal materials.
Co-founder Dai Yinrong serves as a foreign communication academician of the French National Academy of Medical Sciences and an academician of the Chinese Academy of Engineering, and is currently a tenured professor of the Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, the director of the Shanghai Joint Surgery Clinical Medical Center, the director of the Engineering Research Center of Digital Medicine Clinical Translation Ministry of Education and the director of the 3D Printing Technology Clinical Translation R&D Center, and the director of the Stem Cell and Regenerative Medicine Transformation Base of the Shanghai Jiaotong University Translational Medicine Research Institute. At the same time, Dai Established the first orthopedic biomechanics research laboratory in a domestic hospital.
The birth of Senfeng Medical just combines Yang Ke's advantages in engineering with Dai Yinrong's advantages in the field of medicine. As a result, the orthopedic implant consumables market, which urgently needs new technologies, new materials and new processes, can continue to have emerging products.
Not only that, but the company's core team members are also quite understanding of the supply chain. Wei Xiang, general manager of Senfeng Medical, and Yu Yachuan, director of commercial development, jointly made supplier integration, and from a management point of view, we have standardized each process including process flow chart to ensure the stable quality of raw materials and sufficient production capacity.
In the orthopedic implant market, Senfeng Medical seems to be ready for both technology, raw materials and talent. Senfeng Medical, which has mastered new processes, new materials and new technologies, undoubtedly has more possibilities for development in the medical device market in the future.
At the end of the conversation, Yu Yachuan said: "We plan to obtain three types of registration certificates for antibacterial metal material orthopedic implants in the short term, and promote the antibacterial metal material from the current group standard of the Biomaterials Association to the industry standard, and promote the wider application of materials in the industry." ”