With the development of electric vehicles for many years, the mileage that consumers often hang on their lips has always been the biggest challenge for car companies in the industry. From 150km to 300km, 500km, 700km progress brought about by the results are also more and more obvious, especially in recent years, silicon anode, CTP, CTC and other technologies related to power batteries have landed, adding a boost to the endurance of the vehicle, but these technologies are still strange in the eyes of ordinary users. Therefore, this issue of E said to understand to analyze the development of pure electric vehicle power batteries for you, so that you can make an introductory understanding of these technologies.
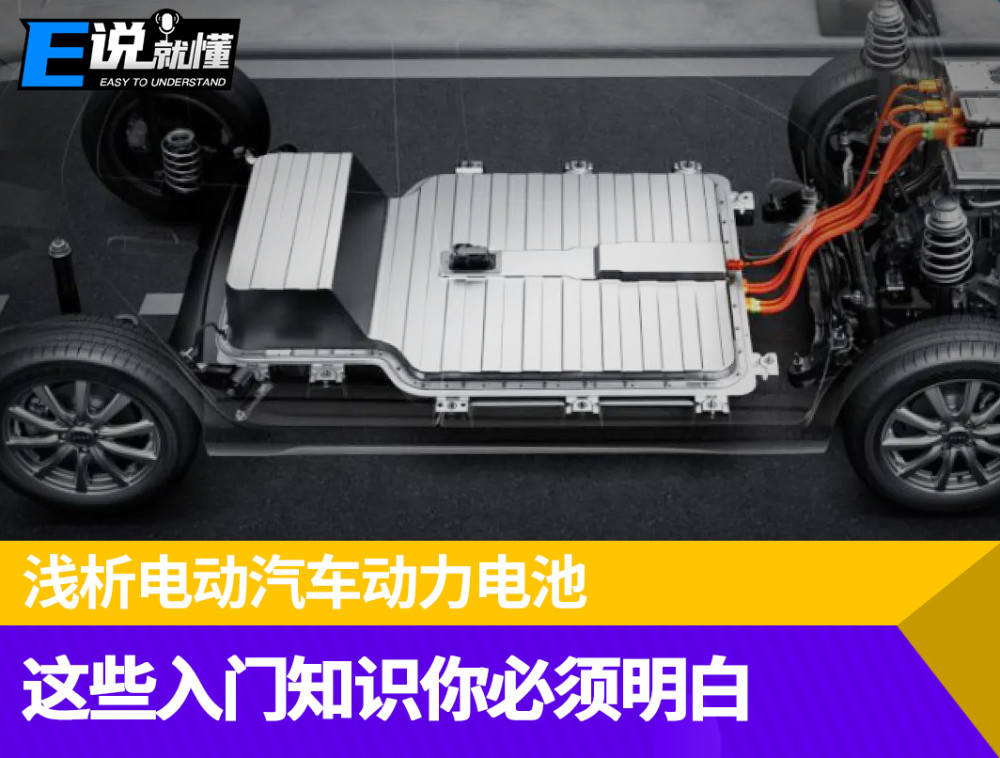
Throughout the history of the development of power batteries, there are basically only two development routes, I call it "chemical means" and "physical means", that is, the evolution of battery materials and battery loading technology, the two means have their own strengths, let's talk about their respective characteristics in detail.
"Chemical means" - the evolution of battery materials
At present, the power batteries we often hear now are divided into: lithium cobalt oxide (LiCoO2), lithium manganate (LiMn2O4), lithium nickel cobalt manganese oxide (LiNiMnCoO2 or NMC), lithium nickel cobalt aluminuminate (LiNiCoAlO2 or NCA), lithium iron phosphate (LiFePO4), lithium titanate (Li4Ti5O12). Among them, lithium iron phosphate and nickel-cobalt manganese oxide (ternary lithium) are more common to us. However, although the material elements are different, the overall internal structure of the lithium battery is the same. In general, the structure of the battery is divided into positive electrode material, electrolyte, diaphragm, and negative electrode material in order. The aforementioned lithium iron phosphate and lithium nickel cobalt manganese oxide are both positive electrode materials for batteries, that is, standards for positioning different categories of batteries, while the negative electrode materials are graphite or silicon.
So, how does it work? Simply put, the entire operation process of lithium batteries can be said to be a "migration" process of lithium ions.
When the positive electrode material of the battery generates lithium ions, these lithium ions "swim" into the electrolyte from the positive electrode, through the electrolyte "through" the bending hole in the diaphragm, moving to the negative electrode, that is, embedded lithium, and the electrons that have long run through the external circuit to the negative electrode are combined to ensure the balance of the positive and negative charges, and the electrons running outside are the electrical energy we use. Therefore, because the negative electrode material graphite is a multi-layer structure, it can store lithium ions between the layers, just like the refrigerator can store food.
In general, the cathode material of the battery is an important criterion for determining the overall energy density and temperature resistance of the battery. Earlier we mentioned the common lithium iron phosphate and ternary lithium batteries on the market, and which of these two battery cathode materials is superior or inferior? I'll explain them one by one from a few points.
Energy density: lithium iron phosphate < ternary lithium
Lithium iron phosphate battery full name lithium iron phosphate lithium battery, refers to lithium iron phosphate as the cathode material lithium ion battery. The full name of ternary lithium batteries is "lithium-ion batteries with cathode materials using ternary polymers such as nickel-cobalt manganese oxide or nickel-cobalt-aluminum oxide." Among them, the lithium iron phosphate battery due to the elemental structure caused by the low gram capacity and voltage platform, and the lithium iron phosphate particles themselves are not dense, resulting in its low vibration density and compaction density. That is to say, under the same volume conditions, lithium iron phosphate is less, the natural capacity is small, and the energy density is low.
The ternary lithium battery is composed of nickel, cobalt, and manganese, of which: nickel can improve the reversible capacity of the material, and determines the gram capacity of the internal material of the battery (the theoretical gram capacity of lithium iron phosphate is only 160mAh/g, while the ternary material nickel cobalt manganese (NCM) is about 200mAh/g. Therefore, the ternary material battery can have a higher battery energy, just like a player with full body muscles is more energetic than ordinary people, but if its content is too high, the circulation performance of the material will become worse.
Cobalt can make the de-embedding of lithium ions easier, improve the conductivity of the material and improve the discharge cycle performance, but the price of cobalt is relatively high, especially since this year, the price of cobalt has risen from less than 500,000 yuan per ton to about 550,000 yuan per ton, so too much content will lead to increased costs and reduce cost performance. Manganese can improve the safety and stability of the material, but too high a content will reduce the gram capacity of the material.
Therefore, at present, many companies are developing 811 high-nickel batteries (nickel-cobalt-manganese ratio of 8:1:1), improve the nickel use content, then the energy density of ternary batteries will also increase, but the thermal stability has declined.
Low temperature performance: lithium iron phosphate < ternary lithium
As we all know, lithium iron phosphate batteries have lower performance than ternary lithium batteries under low temperature conditions, why is this?
First of all, the conductivity of lithium iron phosphate material at room temperature can be lower than that of ternary materials by 4 orders of magnitude, especially at -20 ° C, the capacity of lithium iron phosphate batteries can only reach 1/3 of normal temperature, and the lithium ion diffusion coefficient in it is two orders of magnitude lower than that of normal temperature, and when the temperature continues to drop to -40 ° C, lithium iron phosphate can only maintain 20% of the normal temperature capacity. This is mainly because the adjacent FeO6 octahedron in the structure of the lithium iron phosphate battery is connected through the co-apex, and the conductivity of this structure is very low, so the diffusion rate of lithium ions in the material is very slow, so the charge and discharge efficiency is affected. In addition, in the low temperature environment, the material activity is reduced, and the number of lithium ions that can move is reduced, resulting in poor low temperature performance. Ternary materials do not have this problem, so in the low temperature environment, charging and discharging are less affected.
However, here to interject, affecting the low temperature performance of the battery in addition to the difference in the cathode material, another reason is the electrolyte. Due to the presence of a high melting point solvent in the electrolyte, and it will produce a certain solidification phenomenon when the temperature is too low, and as mentioned above, the process of charging and discharging the ion battery is the process of lithium ions moving back and forth between the positive and negative electrodes of the battery through the electrolyte. So when the electrolyte begins to viscous and solidify under low temperature conditions, the resistance of the lithium-ion battery to move in the electrolyte becomes larger, just like before swimming in the pool filled with water, now the water has become silt, thereby reducing the movement speed of lithium ions, resulting in some lithium ions even unable to penetrate the battery separator to complete the de-embedding and embedding of the positive and negative electrodes, so that the battery charge and discharge is reduced.
Safety performance: lithium iron phosphate > ternary lithium
In terms of battery safety, the P-O bond in the lithium iron phosphate crystal is very stable and difficult to decompose, so even at high temperature or overcharge, it will not collapse like lithium cobalt oxide or form a strong oxidizing substance, and the decomposition temperature of lithium iron phosphate is about 600 ° C, so it has good safety. Although in the case of overcharge, there has been combustion and explosion, but its overcharge safety has been greatly improved compared with ordinary liquid electrolyte lithium cobalt oxide batteries and ternary batteries.
The ternary lithium material will decompose at about 200 degrees. And the chemical reaction is more intense, will release oxygen molecules, under the action of high temperature electrolyte rapid combustion, more will occur chain reaction. Lithium iron phosphate will only decompose at 700-800 degrees, will not release oxygen molecules like ternary lithium materials, and the combustion is not so intense.
Silicon carbon anode material
In addition to the positive electrode material, the negative electrode material is also another criterion that determines the energy density of the battery. At present, our common batteries use graphite as the negative electrode material, which has certain limitations in storing lithium ions, only 372mAh/g. Therefore, how to increase the capacity of the battery has become the key to changing the endurance of electric vehicles. Therefore, in the continuous search and search, the material of silicon was finally found.
In terms of energy storage characteristics, the energy storage capacity of silicon is more than 10 times that of graphite, reaching 4200mAh/g, and the service life of lithium-ion batteries with silicon electrodes is about 30% longer than that of lithium-ion batteries with graphite electrodes.
However, although the silicon element has a large capacity, it is very easy to expand, and the volume change of the silicon material in the reaction is as high as 320%, which is much greater than the volume change of the existing carbon material by 12%, which not only leads to the pulverization and fragmentation of the silicon material particles, causing the destruction and regrowth of the SEI film, consuming limited lithium ions. In addition, it will also destroy the negative conductive network, resulting in some active substances can not participate in the reaction, resulting in a rapid decline in the reversible capacity of the negative electrode containing silicon materials, so to apply silicon to the battery anode material, the technical strength of the enterprise is very tested.
Solid-state batteries go to war!
Now we know that the lithium iron phosphate battery or ternary lithium battery used in new energy vehicles now belongs to the liquid battery because it contains a large amount of electrolyte. However, due to the characteristics of the material, the electrolyte cannot inhibit the formation of lithium crystal branches, the safety performance is poor, and the low temperature effect is not good, so the all-solid-state battery is born.
The biggest difference between solid-state batteries and current mainstream traditional lithium-ion batteries is the electrolyte. Solid-state batteries use solid electrolytes, replacing the electrolyte and diaphragm of traditional lithium-ion batteries, working at large currents will not puncture the diaphragm due to the appearance of lithium dendrites, causing short circuits, will not occur at high temperatures, and will not burn due to gas production. And after the all-solid-state electrolyte, the battery can not use the graphite anode embedded with lithium, but directly use metal lithium to do the negative electrode, which can greatly reduce the amount of anode material, so that the energy density of the entire battery has been significantly improved, up to 300-400Wh/kg. In addition, the solid electrolyte solves the problem of the solid electrolyte interface membrane formed by the liquid electrolyte during the charging and discharging process and the lithium dendrite phenomenon, which greatly improves the cycle and service life of the lithium battery, and can reach about 45,000 cycles.
However, there are always good and bad sides to everything, although solid-state batteries have various benefits, but the following points are the main reasons that restrict their development.
First of all, because of the use of solid electrolytes, so there is a connection between it and the electrode material in a solid state, resulting in a weak effective contact between the electrode and the electrolyte, and the transmission kinetics of ions in solid matter are low, which will cause the problem of excessive interfacial impedance.
Secondly, the choice of electrolyte for solid-state batteries is also a very tricky difficulty. There are four known development routes: polymers, films, sulfides and oxides. Among them, for thin-film solid-state batteries and oxide solid-state batteries, it is difficult to develop large-capacity power or energy storage batteries; polymer solid-state batteries are limited by the existing polyethylene oxide material system, cannot work at room temperature and are difficult to be compatible with high-voltage positive electrodes; sulfide solid-state batteries face technical problems such as electrolyte sensitivity to air, harsh manufacturing conditions, expensive raw materials, and immature large-scale production technology.
Finally, there is the cost of solid-state batteries. First of all, the production process of all-solid-state batteries is very different from the liquid batteries we commonly see now, so it cannot be produced in a collinear manner. Therefore, if you choose to produce and manufacture solid-state batteries, you need to redesign and build a set of production lines, and the price of solid-state electrolytes is also very expensive, so a series of factors lead to the cost of all-solid-state power batteries at this stage is still high, which also leads to many battery manufacturers to retreat to the second, the liquid battery and solid-state battery mixed in the battery pack, forming a semi-solid-state battery with economical price and energy density.
However, it can be seen that the ultimate form of the new energy vehicle battery should be a solid-state battery, but it cannot be enlarged under the constraints of production cost and battery technology, so it is still relying on lithium iron phosphate and lithium ternary to support the scene, and these two to today's technological development to enter the "ceiling" state, so how to improve the battery life without changing the battery material? Thus the "physical means" appeared.
"Physical means" - the evolution of battery modules
In fact, the common pure electric vehicles on the market are the same as the electric toy cars we played with when we were young, and the power source comes from the batteries under the body, but the batteries of pure electric vehicles are larger and more complex. The battery system of the early electric vehicle model consisted of a battery cell, a module, and a battery pack. First of all, there are multiple cells to form a battery module, and then a complete battery pack is composed of multiple battery modules. It's like putting bagged coffee into a box and then stuffing multiple boxes full of bagged coffee into large boxes for shipment. And in these battery modules, there will also be some pipeline wires distributed for cooling and transmission work, is it not complicated?
However, this battery system due to the use of modularization, so itself in the structure of some space waste, its pipeline and module box and so on occupy the volume, so that the battery with capacity only occupies about 50% of the overall battery pack internal space, so at that time you see the pure electric vehicle although the battery pack is huge, but it can only run 200km. However, at that time, this was also a helpless move, because the pure electric vehicle had just started, so the performance of the battery cell was not stable, so in order to effectively avoid problems such as thermal runaway, the module design had to be adopted.
As a result, with the development of electric vehicle technology, CTP technology to remove the module was born.
The full name of CTP is cell to pack, that is, the technology of integrating the battery cell directly into the battery pack, just like the bag coffee mentioned above is placed directly into a large box. Battery packs using this technology eliminate the design of the battery module, reduce battery costs, and increase the energy density of the battery pack.
Here you should ask, why can we increase the energy density? Isn't it up to the battery to decide?
In fact, no, here I first list a formula: battery pack energy density = cell energy density× group efficiency.
To achieve a high energy density at the battery pack level, in addition to improving the quality of the battery cells, it is also very important to improve the efficiency of the group. Usually, traditional power batteries are composed of three layers of structure, namely cell modules and battery packs, and their general group efficiency is 60% to 70%. In other words, you buy a house, and this 60%-70% is only your use area, and the rest is the pool area. Therefore, if you add the power loss caused by components such as pipelines, the energy density of a complete set of battery packs is lower than the energy density of the cells.
According to the data, the energy density of a domestic brand of batteries exceeds 300Wh/kg in the single body, but it is limited by the grouping method of traditional battery packs, and the energy density at the battery system level is still about 160Wh/kg. Therefore, reducing the "unnecessary" components in the battery pack to plug more cells to improve the efficiency of the group, and also to ensure the mechanical strength of the battery pack level frame, BMS and thermal management capabilities, this technology trend is called de-modularization, that is, CTP technology.
CTP technology can eliminate or reduce the assembly of module end plates, side plates, pipelines and screws for fixing the module and other fasteners, can improve the volume utilization rate, so due to the reduction of the internal structure of the battery pack, so the overall weight is also reduced, the mass energy density is also improved to increase the endurance. And because the assembly process of the battery is simpler, the manufacturing costs such as manpower and material resources are saved, and the cost of the parts is reduced, and the cost of the battery pack will also be reduced.
However, this battery technology also has certain limitations. First of all, after the lack of modules and some components, the overall support strength of the battery pack will face major challenges, and after the module design is missing, the prevention of battery cell thermal runaway system configured above will also be cancelled, so the requirements for battery BMS control strategies are also more stringent.
Another point to say is that CTP technology will have higher requirements for cell consistency, so what does this consistency mean?
First of all, we must first understand a theory called the "barrel effect", how much water a barrel can hold, does not depend on the longest plank, but on the shortest board.
The same goes for battery packs. Taking the early module battery as an example, a single cell is formed by parallel or series connection to form a battery pack. No matter how good the performance and quality of a single cell is, if the characteristics of each single cell in the same group are inconsistent or the initial state of the combination package is inconsistent, it will lead to the performance of each single battery not being fully utilized, and the phenomenon of mutual "containment" or "dragging legs" between the single batteries will occur, which will cause problems such as capacity loss, life reduction and internal resistance increase, so that the overall characteristics of the battery pack will decline sharply or some batteries will be damaged at an accelerated rate.
In addition to CTP technology, in order to further improve the endurance and eliminate the "unnecessary" battery pack components, some car companies have issued a new CTC technology (Cell To Chassis), which is the battery chassis integration technology. This technology can actually be seen as an "extreme", it basically does not even need the battery pack, the battery is directly placed on the chassis, that is, the members of the car sit directly on the power battery. And the strength of the battery system structure of CTC technology is completely guaranteed by the strength of the battery shell and the strength of the body, so this will have more demanding requirements for the production of batteries.
On the whole, although CTP technology or CTC technology will improve the energy density of the battery pack, there will be challenges in terms of safety, especially whether consumers can accept this technology in terms of psychology. In addition, due to the cancellation of the module design, if a single cell fails, the entire battery pack can only be removed in repair, so the maintenance cost will be more, but with the future development, I believe that in the future, systematic changes will be made in the later maintenance of this technology.
Write at the end:
The development of modern new energy vehicles is actually the last two decades, from a niche "supporting role" to today's "protagonist" position, the development and progress of battery technology is indispensable. The content of this issue of the author in-depth and simple list of the mainstream development of battery technology in the market, but from the perspective of technical macro, these technologies are still only rare, sodium-ion batteries, graphene batteries, etc. are accompanied by the tiger, but because the existing technology is not popularized, so the author will not repeat it too much. But it is certain that the technological breakthrough of the power battery in the future may flourish in the controversy of car companies, and it is definitely a positive thing for us consumers. After that, EV Vision will also popularize more knowledge about new energy vehicles, so stay tuned.