Abstract: A new method for realizing the coupling reaction of indole itself is proposed by anhydrous aluminum trichloride instead of protonic acid or acid anhydride as a catalyst. The method uses an inexpensive catalyst, is easy to operate, has a high yield (86%-90%), and has a short reaction time (less than 40min). The atomic utilization rate of this reaction is 100%, which has certain applications in organic synthesis. The structure of the product was characterized by NUCLEAR MAGNETIC RESONANCE hydrogen spectroscopy and NMR carbon spectroscopy. In addition, the possible reaction mechanism is proposed.
introduction
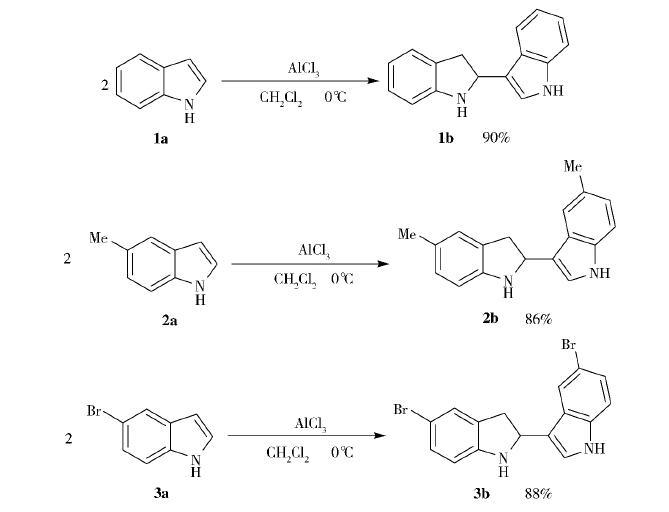
Indole (2,3-benzopyrole) is an important nitrogen-containing heterocyclic compound, which is an important intermediate in organic synthesis and has a wide range of applications in medicine, pesticides, spices, food, feed additives and dyes. Due to its unique biological activity and pharmacological effects, indole has become a star molecule at the intersection of biology, medicine and materials, and has been attracting chemists to modify its structure and conduct biomimetic fully integrated research for many years. Indole ring-containing compounds in nature are widely distributed, with more than 3,500 species discovered since 1983, many of which are importantly biologically active and closely related to life activities. For indole itself, the chemical reaction is also extremely rich, and it can be derived by various methods. Indole molecular structure, C-3 positions are the most reactive and have the most types of reactions associated with them, so indole is the most eye-catching among many derivatives. Relevant literature reports that indole indole in the protonic acid or acid anhydride catalytic action, a self-coupling reaction, the atomic utilization rate of this reaction is 100%, has a very important research value. In 2011, Norio Shibata's research group reported that indole can be mixed overnight under the catalytic action of trifluoroacetic anhydride to produce indole coupling products; in 2013, Xi Zhen et al. reported that indole can also obtain the same product after stirring in ether solution of saturated hydrogen chloride gas for 18 hours. Although both methods have successfully achieved self-coupling of indole, there are still certain drawbacks: (1) a long reaction time (e.g., 18h or overnight) ;(2) the product yield is low (e.g., 63%) ;(3) and the preparation is tedious (e.g., the preparation of a diethyl ether solution of saturated hydrogen chloride gas). Therefore, exploring a suitable reaction condition to achieve the self-coupling of indole is still an urgent problem to be solved. As we all know, anhydrous aluminum trichloride is an economically available, easy to operate, widely used Louis acid catalyst. An attempt is made herein to use anhydrous aluminum chloride instead of hydrogen chloride gas and trifluoroacetic anhydride to catalyze the self-coupling reaction of indole. Experiments show that under the condition of anhydrous aluminum trichloride as a catalyst, 0 °C and dichloromethane as solvents, the reaction can be completed within 40 minutes, and the synthesis of three indole self-coupling products is finally realized, and the yield can reach 86%-90%. The reaction equation is:
Edit toggle to center
Add a picture annotation of up to 140 words (optional)
1 Experimental section
1. 1 Main instruments and reagents
Instruments: 50mL round-bottom flask, magnet, cassette ultraviolet analyzer (ZF-20D), INOVA-400MHz nuclear magnetic resonance instrument (American company), BL 120P electronic analysis balance, IKA (RH basic 1), SHZ-D(III.) type circulating water vacuum pump, RE-2000A rotary evaporator.
Reagents: The reagents used in the experiment are commercially available for analytical purity.
1. 2 Synthesis steps
In a 50mL round-bottom flask that has been placed into the magneton, the 10mmol reaction substrate is dissolved in 20mL dichloromethane, and then the round-bottom flask is placed in an ice bath to cool to 0 °C; under the condition of good ventilation and normal magnet stirring, 12mmol[13] anhydrous aluminum trichloride is added to the round-bottom flask in batches to ensure that the reaction continues at 0 °C; monitor the reaction with TLC thin layer chromatography, when the substrate is fully reacted (less than 40min), slowly inject 0 °C of water into the body and stir continuously, Until the organic layer and the aqueous layer become clear and translucent; the organic layer is separated by a separating funnel and washed with saturated salt water three times, the organic layer is dried with anhydrous sodium sulfate for 20 minutes and then filtered, and the filtrate is concentrated by rotation to obtain a crude product; the crude product is obtained by column chromatography (ethyl acetate / petroleum ether = 1/6) to obtain the corresponding white solid product. 2 Analysis and discussion
2. 1 NmR data analysis
First of all, the number of carbon and hydrogen atoms of the three products can be known from the NMR data: product 1b contains 14 hydrogen atoms and 16 carbon atoms; product 2b contains 18 hydrogen atoms and 18 carbon atoms; product 3b contains 12 hydrogen atoms and 16 carbon atoms. Secondly, it can be seen that the 1 H NMR signals of the three products are similar, and all have five similar characteristic peaks. Take product 1b as an example to illustrate:
It is worth noting that product 2b has two distinct -Me signals in high field compared with 1b and 3b, 1 H NMR and 13C NMR. In addition, the products 2b and 3b have two more solitary groups of 1H NMR in the low field due to the special position of -Me and -Br. In summary, the 1 H NMR and 13C NMR of the three products are consistent with their structure.
2. 2 Reaction Mechanism Discussion The author combines the relevant literature and experimental results to propose a possible reaction mechanism: first, aluminum trichloride activates the C-3 position of indole to generate an imide ion intermediate, so that the C-2 position of indole is positively charged; then another molecule of indole C-3 carbon atoms attack this positive center [17] to achieve coupling; the coupling intermediate undergoes proton transfer [and loses aluminum trichloride to form the final product, and the departing aluminum trichloride continues to catalyze the reaction (Figure 1)."
3 Conclusion
In summary, the self-coupling reaction of a series of indoles is achieved by using Louis acid anhydrous aluminum chloride as a catalyst instead of protonic acid or acid anhydride. The advantages of this new method compared with previous work are: (1) the catalyst is inexpensive and easy to use; (2) the reaction time is short; and (3) the product yield is high. The reaction mechanism proposed in this paper provides a basis for future research on indole-related coupling reactions. At present, the application of this reaction is still under study.