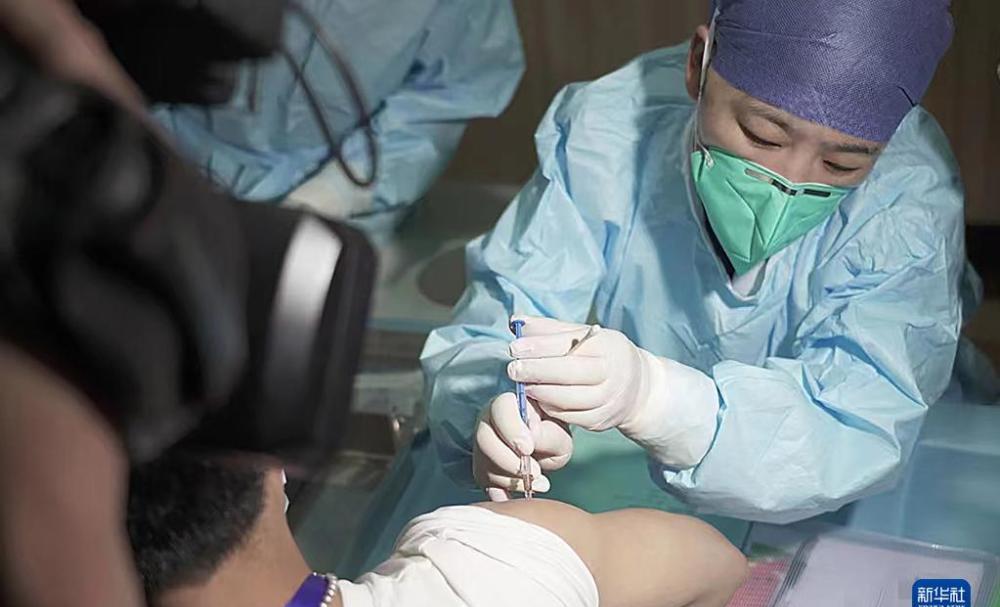
On May 1, a clinical trial volunteer injected the Inactivated Vaccine of the New Coronavirus variant of the Omikejong variant in Hangzhou.
On May 1, the clinical study of the inactivated covid-19 vaccine of the Aumechjong variant strain developed by Sinopharm's China Bio-Beijing Institute of Biological Products was officially launched in Hangzhou, Zhejiang Province. After relevant testing, informed consent, basic physical examination, blood sampling and other links, the volunteers of the clinical trial were vaccinated with the new coronavirus vaccine of the Aumi Kerong variant at Shulan (Hangzhou) Hospital.
On April 26, Sinopharm's Inactivated Vaccine for the New Coronavirus of China Biological Aomi Kerong Variant Was Approved by the State Food and Drug Administration, which means that the vaccine will enter the clinical trial stage.
Xinhua News Agency
On May 1, a clinical trial volunteer injected the Inactivated Vaccine of the New Coronavirus variant of the Omikejong variant in Hangzhou.
On May 1, the clinical study of the inactivated covid-19 vaccine of the Aumechjong variant strain developed by Sinopharm's China Bio-Beijing Institute of Biological Products was officially launched in Hangzhou, Zhejiang Province. After relevant testing, informed consent, basic physical examination, blood sampling and other links, the volunteers of the clinical trial were vaccinated with the new coronavirus vaccine of the Aumi Kerong variant at Shulan (Hangzhou) Hospital.
On April 26, Sinopharm's Inactivated Vaccine for the New Coronavirus of China Biological Aomi Kerong Variant Was Approved by the State Food and Drug Administration, which means that the vaccine will enter the clinical trial stage.
Xinhua News Agency
On May 2, clinical trial volunteers were vaccinated against the New Coronavirus inactivated vaccine against the Omiljung variant in Hangzhou.
On May 1, the clinical study of the inactivated covid-19 vaccine of the Aumechjong variant strain developed by Sinopharm's China Bio-Beijing Institute of Biological Products was officially launched in Hangzhou, Zhejiang Province. After relevant testing, informed consent, basic physical examination, blood sampling and other links, the volunteers of the clinical trial were vaccinated with the new coronavirus vaccine of the Aumi Kerong variant at Shulan (Hangzhou) Hospital.
On April 26, Sinopharm's Inactivated Vaccine for the New Coronavirus of China Biological Aomi Kerong Variant Was Approved by the State Food and Drug Administration, which means that the vaccine will enter the clinical trial stage.
Xinhua News Agency
The inactivated vaccine of the Omiljung variant strain developed by Sinopharm's Beijing Institute of Biological Products on April 22.
On May 1, the clinical study of the inactivated covid-19 vaccine of the Aumechjong variant strain developed by Sinopharm's China Bio-Beijing Institute of Biological Products was officially launched in Hangzhou, Zhejiang Province. After relevant testing, informed consent, basic physical examination, blood sampling and other links, the volunteers of the clinical trial were vaccinated with the new coronavirus vaccine of the Aumi Kerong variant at Shulan (Hangzhou) Hospital.
On April 26, Sinopharm's Inactivated Vaccine for the New Coronavirus of China Biological Aomi Kerong Variant Was Approved by the State Food and Drug Administration, which means that the vaccine will enter the clinical trial stage.
Xinhua News Agency