▎ WuXi AppTec content team editor
On February 8, 2022, Eureka Therapeutics announced that the U.S. FDA has granted orphan drugs for the treatment of hepatocellular carcinoma (HCC) for the treatment of T cell therapies ET140203 and ECT204 under investigation. Both T cell candidates were developed based on its proprietary ARTEMIS technology platform, targeting specific hepatic cancer antigens. Eureco Bio is currently recruiting patients in three Phase 1/2 clinical trials to evaluate their safety and potential efficacy, respectively.
Liver cancer is one of the leading causes of cancer-related deaths worldwide. It is estimated that there will be more than 905,000 new cases of liver cancer worldwide in 2020, and more than 830,000 people will die from the disease. HCC is the most common type of liver cancer, accounting for about 75% to 90% of primary liver cancer cases. Worldwide, the main factors that increase the risk of HCC are chronic hepatitis B and chronic hepatitis C, alcohol consumption, and metabolic syndrome.
The ARTEMIS platform has two core functional components: an antibody-based antigen-binding domain and an effector domain. ARTEMIS's receptor design allows it to take advantage of the activation and regulation pathways commonly employed by endogenous T cell receptors (TCRs). Because this strategy does not directly connect the intracellular signal domain to the co-stimulation domain, it is expected to eliminate cytokine release syndrome (CRS) caused by overactivation of T cells.
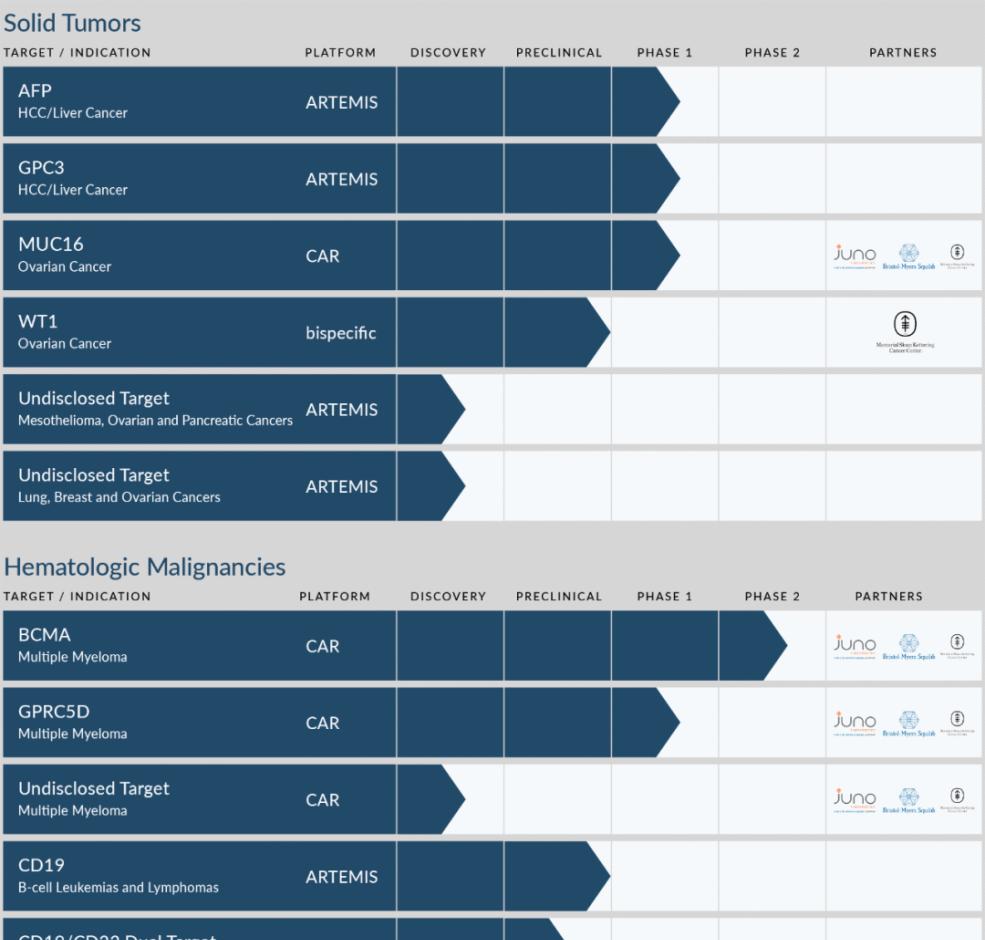
▲Eureco Biological Product Pipeline (Image source: Euryco Biological Company official website)
The treatment process of ET140203 involves the collection of patient's T cells, which are engineered to express Eureco's proprietary ARTEMIS cell receptor and infused back into the patient. Receptors expressed by modified ET140203 T cells target alpha-fetoprotein (AFP)-peptide/HLA-A2 complex expressed on liver cancer cells. In addition, et140203 T cells have integrated Euryco Bio's proprietary tumor infiltration technology, which has been demonstrated in animal models to improve the ability of drugs to infiltrate solid tumors, which is expected to improve patient efficacy. The therapy is currently being evaluated in two open-label, dose-escalating, multicenter Phase 1/2 clinical trials, one in adult patients with AFP-positive HCC and one in pediatric subjects with AFP-positive patients with relapsed/refractory hepatoblastoma (HB), unspecified hepatocellular tumors (HCN-NOS), and HCC.
ECT204 targets GPC3 expressed on liver cancer cells, a promising HCC antigen present in more than 70% of HCC cells. ECT204 T cells also incorporate Eureco Bio's proprietary tumor infiltration technology. It is currently being evaluated in a Phase 1/2 clinical trial for the treatment of adult patients with GPC3-positive HCC.
▲Dr. Liu Cheng, CEO of Euryco Biologics