DNA nanomachines are nanostructures formed based on the self-assembly of DNA. By embedding multiple types of functional nucleic acid units, DNA nanomachines can initiate structural changes in response to endogenous stimuli in tumor cells (e.g., glutathione, microRNA, etc.) to achieve tumor cell imaging or treatment. However, the microenvironment of solid tumors is complex, and there are a large number of healthy cells with important functions (such as fibroblasts, immune cells, etc.) around tumor cells. Conventional DNA nanomachines are often mistakenly activated before they enter targeted tumor cells due to the influence of endogenous stimuli in the solid tumor microenvironment, causing toxic side effect damage to surrounding healthy cells. Therefore, the development of a nanomachinechanic that is specifically activated at the cellular level is of great significance for the precise treatment of tumors.
Cell membrane is a natural barrier that separates cells from the external environment, and the binding of nucleic acid aptamers to membrane proteins enables DNA nanomachines to perform logical operations on the surface of the cell membrane and generate output signals. In the previous study, Ju Xianxian and Professor Liu Ying of the State Key Laboratory of Life Analytical Chemistry reported a "double-lock-smart key" DNA logic gate model based on nucleic acid aptamer and double receptor binding on the cell membrane, and achieved a distinction between cell subtype specificity in tumor cell recognition and tumor therapy (Nat. Commun. 2016, 7, 13580)。 However, completing the "logic gate" operation on the surface of the cell membrane relies on the migration of DNA strands on the cell membrane. This process relies on the "shedding and recombination" of DNA strands, which is limited in efficiency and prone to false positive results. In order to solve this problem, the research team designed a nano-machine (Figure 1a) that performs DNA logic gate operations across cell membranes, and its two-step DNA operations are completed on the cell membrane and in the cytoplasm, which can avoid the non-specific activation of DNA nanoprobes in the solid tumor microenvironment, and realize the precision photodynamic therapy of solid tumors in living organisms. This DNA nanomachineograph consists of upconversion nanoparticle cores (UCNPs), DNA assemblies L012, and H012 (Figure 1b). The DNA strand LA-apt containing sgc8 aptamer binds to the overexpressed PTK-7 protein on the cancer cell membrane to form a signal input terminal1, and the highly expressed miRNA-21 in cancer cells is used as the signal input terminal 2. These two signal inputs, together with the L012 section on the DNA nanomachinechanic, form the "AND" logic gate.
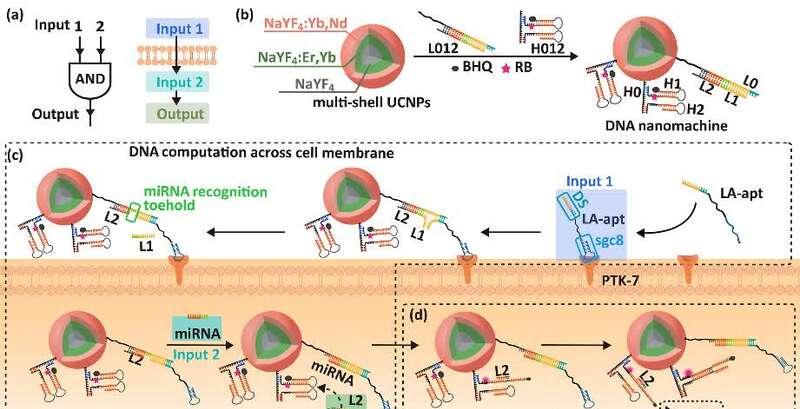
Figure 1. (a) DNA transmembrane logic gate operations and (b) schematic diagrams of DNA nanomachines. (c) L2 is released by transmembrane operations using LA-apt and PTK-7 protein binding to form signal input terminal 1 and miRNA-21 as signal input terminal 2. (d) L2 initiates a cyclic reaction to restore photosensitizer activity.
The process of transmembrane logic computation by DNA nanomachines is as follows: LA-apt hybridizes with L012 on the surface of the cell membrane, replaces the L1 strand and exposes the binding site of miRNA-21, and induces the nanoparticles to enter the cytoplasm; miRNA-21 in the cytoplasm reacts with L012 on the nanoparticles, outputting a signal (i.e., the released L2 strands) (Figure 1c); And then open the H2 issuing card to re-release the L2 chain for the next cycle. This process continuously restores the activity of the photosensitizer, producing reactive oxygen species under the excitation of the green emitted light of the UCNPs, enabling photodynamic therapy of tumor cells (Figure 1d).
Modification of ros indicator DHR123 on DNA nanomachines can verify intracellular precision photodynamic therapy. After LA-apt is anchored on the cell surface, UCNPs-DNARB/BHQ-incubated MCF-7 cells have significant ROS production under near-infrared illumination (Figure 2a), while their controls cannot be ROS produced (Figure 2b). UCNPs-DNA'RB/BHQ, a control material with unblocked miRNA recognition sites, showed significant ROS fluorescence in miRNA-21-negative MCF-7 cells after incubation with miRNA-21, while UCNPs-DNA RB/BHQ did not produce ROS in this cell (Figure 2c). Therefore, blocking the miRNA recognition site ensures that photodynamic therapy occurs only within tumor cells and avoids damage to normal cell tissue, thereby achieving accurate and efficient photodynamic therapy for cancer cells (Figure 2e).
Figure 2. (a) CONFOC IMAGING OF LA-APT and (b) LA'-APT-ANCHORED UCNPs-DNARB/BHQ after treatment of MCF-7 cells. (c) CONFOC IMAGING OF LA-APT-ANCHORED MIRNA-21-NEGATIVE MCF-7 CELLS AFTER TREATMENT WITH UCNPs-DNARB/BHQ or UCNPs-DNA'RB/BHQ. (d) Cell viability of MCF-7 control cells versus MCF-7 cells treated in different ways. (e) Cell proliferation of MCF-7 control cells, LA'apt-apt anchored MCF-7 cells after UCNPs-DNARB/BHQ (LA-apt+A), UCNPs-nrDNARB/BHQ (LA-apt+B) or UCNPs-DNA (LA-apt+C) treatment, LA'apt-apt anchored CELLS after UCNPs-DNARB/BHQ treatment (LA'-apt+A).
The effects of DNA nanomachines in photodynamic therapy have also been validated at the animal level (Figure 3). By labeling Cy3 and Cy5 on the surface of UCNP and at the end of the L0 chain, respectively, the two-color nanomachine UCNPsCy3-DNA BHQ-Cy5 was obtained, and the BHQ modified at the end of the H1 hairpin card quenched the Cy3 fluorescence. Activation of DNA nanomachines is revealed by Cy5 fluorescence recovery in living organisms through Cy5 delivery traces of DNA nanomachines. From the mouse fluorescence imaging, it can be seen that although the nanomaterial will partially enter the liver, only the tumor has obvious Cy3 fluorescence, proving that the transmembrane operation of this DNA nanomachine is activated only in tumor cells and does not cause damage to normal organ tissues.
Figure 3. Injection of LA-apt or LA'-apt, followed by UCNPsCy3-DNA BHQ-Cy5 or UCNPsCy3-DNA'BHQ-Cy5 mice with (a) in vivo fluorescence imaging and (b) imaging of organs and tumor tissues.
The above-mentioned results were published on September 2 at the Journal of the American Chemical Society (DOI: 10.1021/ jacs.1c06361) published online. PhD students Zhang Yue and Associate Researcher Chen Weiwei are the co-first authors of the work, and Professor Ju Yixian and Professor Liu Ying are the co-corresponding authors.
In recent years, the research team has made a series of innovative achievements around the "development of upconversion nanoprobes and their performance research in the efficient and accurate diagnosis and treatment of cancer". Upconversion nanomaterials with luminescence enhancement "energy concentration domain" structure (Angew. Chem. Int. Ed. 2019, 58, 12117-12122), developed a relayed energy transfer mode to improve energy transfer efficiency (CCS Chem. 2021, 3, 1510-1521), realizing upconversion luminescence-driven DNA azobenzene nano pumps for controlled release of chemotherapy drugs (Angew. Chem. Int. Ed. 2019, 58, 18207-18211) and near-infrared light-responsive microRNA amplifiers for early-stage cancer precision photodynamic therapy (Angew. Chem. Int. Ed. 2020, 59, 21454-21459), proposed siRNA delivery and gene therapy strategies for near-infrared light-controlled tearing upconversion nanocapsules (Biomaterials 2018, 163, 55-66), which instantly ignited the upconversion nanobombs by near-infrared light, achieving rapid drug release and efficient treatment of cancer (J. Control. Release 2021, 336, 469-479)。
These series of work have been supported by the National Natural Science Foundation of China (21890741), key projects (21635005), surface projects (21974064), outstanding youth fund (22022405), youth fund (22004062), Jiangsu Natural Science Foundation Outstanding Youth Fund (BK20200010), double innovation talent fund, and the State Key Laboratory of Life Analytical Chemistry.
Source: Nanjing University