*For medical professionals only
Among the chemicals involved, the largest number of reports was for tumor drugs, followed by anti-infective drugs
On March 30, 2022, the National Adverse Drug Reaction Monitoring Center organized the release of the National Adverse Drug Reaction Monitoring Annual Report (2021) (hereinafter referred to as the Report).
Adverse drug reactions refer to harmful reactions that occur under the normal dosage of qualified drugs and are not related to the purpose of medication. Adverse drug reactions are inherent properties of drugs, and in general, all drugs will have more or less, light or severe adverse reactions.
The report pointed out that among the chemical drugs involved in the 2021 adverse drug reaction/event report, the top 5 categories in terms of number of cases were tumor drugs, anti-infective drugs and cardiovascular system drugs, analgesics, and digestive system drugs.
The detailed report is described below:
Adverse drug reactions/events reported
1
More women than men had adverse reactions, with intravenous administration accounting for 90%
1.1 Reporting of adverse drug reactions/events in 2021
In 2021, the National Adverse Drug Reaction Monitoring Network received 1.962 million copies of the Adverse Drug Reaction/Event Report Form. From 1999 to 2021, the National Adverse Drug Reaction Monitoring Network received a cumulative total of 18.83 million adverse drug reaction/event report forms (Figure 1).
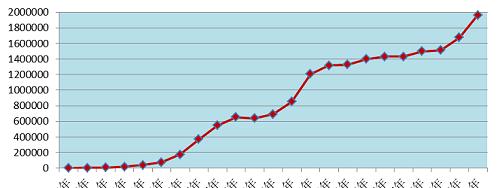
Figure 1 The growth trend of the number of adverse drug reactions/events reported nationwide from 1999 to 2021
1.2 Reporting of new and serious adverse drug reactions/events
In 2021, the National Adverse Drug Reaction Monitoring Network received 597,000 new and serious adverse drug reaction/event reports; new and serious adverse drug reaction/event reports accounted for 30.4% of the total number of reports in the same period.
In 2021, the National Adverse Drug Reaction Monitoring Network received 216,000 reports of serious adverse drug reactions/events, and serious adverse drug reactions/event reports accounted for 11.0% of the total number of reports in the same period (Figure 2).
Figure 2 Proportion of new and severe and severe adverse drug reactions/events reported, 2004-2021
1.3 Adverse drug reaction/event reporting relates to the patient's condition
In the 2021 adverse drug reaction/event report, there were more women than men, and the sex ratio between men and women was 0.86:1. In terms of age distribution, 8.4% of children under 14 years of age and 31.2% of elderly patients aged 65 years and older (Figure 3).
Figure 3 Adverse drug reaction/event reports in 2021 involve patient age
1.4 Adverse drug reaction/incident reports relate to drug conditions
According to the statistics of suspected drug categories, chemical drugs account for 82.0%, traditional Chinese medicines account for 13.0%, biological products account for 2.0%, and unclassifiables account for 3.0% (Figure 4).
Figure 4 Adverse drug reaction/event reports in 2021 relate to drug categories
According to the statistics of the route of administration, in the adverse drug reaction/event report in 2021, injection administration accounted for 55.3%, oral administration accounted for 37.9%, and other routes of administration accounted for 6.8%. Of the injectable administrations, intravenous administration accounted for 90.5% and other injectable administrations accounted for 9.5% (Figure 5).
Figure 5 Adverse drug reaction/event reports in 2021 involve routes of administration
1.5 Adverse drug reactions/events affecting organ systems
Among the adverse drug reactions/events reported in 2021, the top 3 organ system-affected diseases were gastrointestinal system diseases, skin and subcutaneous tissue diseases, systemic diseases, and various reactions at the site of administration.
Figure 6 Adverse drug reactions/events affecting organ systems in 2021
2
Chemical drugs, biological products: tumor drugs and anti-infective drugs ranked among the best
2.1 General Situation
In the 2021 adverse drug reaction/event report, 2.104 million suspected drugs were involved, of which chemicals accounted for 82.0% and biological products accounted for 2.0%. Reports of serious adverse reactions/events in 2021 involved 278,000 suspected medicines, of which chemicals accounted for 87.7% and biologicals accounted for 4.3%.
2.2 Patient-related situations
In the 2021 adverse reactions/events report on chemicals and biological products, the ratio of male to female patients was 0.87:1, and more women than men. 8.6% of patients under 14 years of age reported and 31.4% of elderly patients aged 65 years and older.
2.3 Situations involving pharmaceuticals
Among the chemical drugs involved in the 2021 adverse drug reaction/event report, the top 5 categories in terms of number of cases are tumor drugs, anti-infective drugs, cardiovascular system drugs, analgesics, and digestive system drugs.
Among the serious adverse drug reactions/events involving chemicals in 2021, the largest number of reported drugs was tumor drugs, accounting for 33.2%, followed by anti-infective drugs with 28.1%. According to the proportion of severe reports in this category, the proportion of severe reports of tumor drugs was the highest, at 43.0%, followed by drugs for the motor system at 19.9%.
Among the biologics involved in the adverse drug reaction/event reports in 2021, cytokines accounted for 71.7%, antitoxins and immune serums accounted for 16.0%, blood products accounted for 0.8%, and diagnostic biologics accounted for 0.2%.
According to dosage form statistics, in the 2021 chemical adverse reaction/event report, the proportion of injections and oral preparations was 59.5% and 34.8%, respectively, and other dosage forms accounted for 5.7%. Among biological products, injections and oral preparations accounted for 83.5% and 0.2% respectively, and other preparations accounted for 16.3%.
2.4 Analysis of the overall situation
Reporting of adverse reactions/events in chemicals and biologics in 2021 did not change significantly compared to 2020. From the perspective of the age of patients involved in adverse reactions, the proportion of children under 14 years old still continued the downward trend of last year, but the decline slowed down, and the safety of children's medication was still good overall; the proportion of elderly patients aged 65 and above continued to rise, suggesting that clinical attention should be paid to the safety of medication in elderly patients.
From the perspective of drug dosage forms, the proportion of injections is still showing a downward trend, and the risk is further reduced. From the perspective of drug categories, the number of anti-infective drug reports ranks first, and its proportion has shown a downward trend for ten consecutive years, and the rational use of anti-infective drugs has shown obvious effects; the proportion of tumor drugs continues to rise, and its serious report composition ratio ranks first, suggesting that clinical needs to continue to strengthen the risk management of such drugs. The proportion of biological product reports has increased, of which the proportion of cytokines has increased relatively prominently, mainly related to the rapid increase in the number of new tumor drugs listed in the PD-1/PD-L1 class in recent years and the inclusion of some varieties in the medical insurance directory.
3
There are many adverse reactions of traditional Chinese medicines such as blood-activating and stasis drugs, pyrolytic drugs and antipyretic dehumidifiers
3.1 General Situation
In the 2021 adverse drug reaction/event report, 2.104 million cases of suspected drugs were involved, of which Chinese medicines accounted for 13.0%; in 2021, serious adverse reactions/event reports involved 278,000 suspected drugs, of which Chinese medicines accounted for 5.1%.
3.2 Patient-related situations
In the 2021 Adverse Reactions/Events Report on Chinese Medicines, the ratio of male to female patients was 0.81:1. Children under 14 years of age accounted for 5.7% and elderly patients aged 65 years and older accounted for 29.3%.
3.3 Situations involving medicines
Among the Chinese medicines involved in the 2021 adverse drug reaction/event report, the top 5 categories in the number of cases were blood-activating and stasis-reducing drugs in blood rational agents (24.5%), antipyretic and detoxification drugs in antipyretic agents (11.7%), anti-humidifiers (7.1%), rheumatic drugs in dehumidifying agents (5.2%), and beneficial qi nourishing drugs in tonics (4.9%). In 2021, the top 5 categories in the number of cases of serious adverse reactions/events reported in traditional Chinese medicines were blood-activating and stasis-reducing drugs in blood-rational agents (39.0%), beneficial qi nourishing drugs in tonics (10.7%), antipyretic and detoxifying drugs in antipyretic agents (8.6%), cold prescription drugs in open agents (6.4%), and yang supplements in tonics (4.2%).
In 2021, according to the statistics of adverse reactions/events of traditional Chinese medicines, injection administration accounted for 27.5%, oral administration accounted for 60.5%, and other routes of administration accounted for 12.0%. Among the injectable administrations, intravenous administration accounted for 97.2%, and other injectable administrations accounted for 2.8%.
3.4 Analysis of the overall situation
Compared with 2020, the number of reports of adverse reactions/events in Traditional Chinese medicine increased in 2021, but the proportion of serious reports decreased. From the perspective of the route of administration, the proportion of injectable drug administration has decreased significantly. From the perspective of drug categories, the number of reports of blood-activating and stasis drugs still ranks first, but the proportion has decreased slightly. From the overall situation, the proportion of traditional Chinese medicines in the overall adverse reactions/event reports in 2021 showed a downward trend, but it is still necessary to pay attention to safe drug use.
4
Monitoring of essential medicines
4.1 Overall situation of national essential drug monitoring
In 2021, the national adverse drug reaction monitoring network received a total of 946,000 adverse reaction/event reports from the varieties included in the National Essential Drugs List (2018 Edition), of which 113,000 were serious reports, accounting for 11.9%. The report covered 88.6% of chemicals and biological products, and 11.4% of proprietary Chinese medicines.
4.2 Analysis of the national essential pharmaceutical chemicals and biological products
There are 417 (categories) in the chemical and biological products section of the National Essential Drugs List (2018 edition). In 2021, the national adverse drug reaction monitoring network received a total of 897,000 reports of adverse reactions/events of national essential pharmaceutical chemicals and biological products, of which 135,000 cases were seriously reported, accounting for 15.0%.
In 2021, the national essential medicine chemicals and biological products adverse reactions/event reports are counted according to drug categories, and the top 5 reports are antimicrobial drugs, cardiovascular system drugs, anti-tumor drugs, hormones and endocrine drugs, and drugs for the treatment of mental disorders; the top 5 organ system diseases are gastrointestinal system diseases, skin and subcutaneous tissue diseases, various nervous system diseases, systemic diseases and various reactions at the site of administration, and various examinations.
4.3 Analysis of proprietary Chinese medicines in national essential medicines
The National Essential Medicines List (2018 Edition) involves a total of 268 varieties of proprietary medicines. In 2021, the National Adverse Drug Reaction Monitoring Network received 115,000 reports of adverse reactions/events of proprietary Chinese medicines in the national essential medicines, of which 5,950 cases were severely reported, accounting for 5.2%. In 2021, among the 7 categories of proprietary Chinese medicines of national essential drugs, the total number of adverse drug reactions/events reported from most to less is internal medicine drugs, orthopedics drugs, gynecological drugs, surgical drugs, otolaryngology drugs, pediatric drugs, and ophthalmic drugs.
The above monitoring data show that the overall situation of national essential drug monitoring in 2021 will remain basically stable.
Monographs
According to the results of adverse drug reaction monitoring and public concern, the adverse reaction reports of anti-infectious drugs, cardiovascular system drugs, metabolic and endocrine system drugs, and injections are analyzed, and the safety risks are suggested as follows:
1. Monitoring of adverse reactions of anti-infective drugs
Anti-infective drugs refer to drugs that have the effect of killing or inhibiting various pathogenic microorganisms, including antibiotics, synthetic antibacterial drugs, antifungal drugs, antiviral drugs, etc., which is one of the most widely used drug categories in clinical practice, and the number of adverse reactions/event reports has always been in the first place, which is the focus of adverse drug reaction monitoring.
In 2021, the National Adverse Drug Reaction Monitoring Network received a total of 551,000 reports of adverse reactions/events of anti-infective drugs, of which 62,000 were severe reports, accounting for 11.2%. The number of anti-infective drug adverse reactions/events reported accounted for 28.1% of the total reported number in 2021.
1.1 Situations involving drugs
In 2021, the top 3 drug categories in terms of the number of adverse reactions/events reported on anti-infective drugs were cephalosporins, quinolones, and macrolides, and the top 3 drug categories in terms of the number of reports of serious adverse reactions/events were cephalosporins, quinolones, and anti-tuberculosis drugs.
In the 2021 anti-infective adverse reactions/events report, injections accounted for 76.3%, oral formulations accounted for 19.8%, and other dosage forms accounted for 3.9%; compared with the overall reported dosage form distribution of drugs, the proportion of injections was high. Serious adverse reactions/event reports were reported in 78.6% of injections, 20.1% of oral formulations and 1.3% of other dosage forms.
1.2 Organ system involvement
In the 2021 AFD Adverse Reactions/Events Report, the overall reported and severely reported adverse drug reactions/events involving organ systems are detailed in Figure 7. Compared with the overall report of anti-infective drugs, the proportion of seriously reported systemic diseases and various reactions at the site of administration, immune system diseases, respiratory, thoracic and mediastinal diseases, and various types of examination composition is significantly higher.
Figure 7 Organ system involvement of anti-infective drug adverse reactions/events in 2021
In the overall report on adverse reactions/events of anti-infective drugs, the top 5 diseases of the organ system involving oral preparations are gastrointestinal system diseases, skin and subcutaneous tissue diseases, various nervous system diseases, hepatobiliary system diseases, systemic diseases and various reactions at the site of administration; the top 5 injection involvement organ systems are skin and subcutaneous tissue diseases, gastrointestinal system diseases, systemic diseases and various reactions at the site of administration, various nervous system diseases, and immune system diseases.
In the report on adverse drug reactions/events of anti-infective drugs, the top 5 organ systems involving oral preparations are skin and subcutaneous tissue diseases, hepatobiliary system diseases, various examinations, metabolic and nutritional diseases, and gastrointestinal system diseases; the top 5 injection involvement organ systems are skin and subcutaneous tissue diseases, systemic diseases and various reactions at the site of administration, immune system diseases, gastrointestinal system diseases, and various examinations.
1.3 Monitoring situation analysis and safety risk tips
In recent years, the proportion of anti-infective adverse reactions/events reported in the overall report has shown a continuous downward trend, indicating that the national measures such as strengthening the management of the use of anti-infective drugs have achieved certain practical results, but the number of reports of serious adverse reactions is still high, indicating that the drug risk of anti-infective drugs still needs to continue to be paid attention to.
2. Monitoring of adverse drug reactions in the cardiovascular system
Cardiovascular system drugs refer to drugs used for the treatment of heart disease, vascular protection, blood pressure and lipid regulation, including blood pressure lowering drugs, antianginal drugs, vasoactive drugs, anti-atherosclerotic drugs, antiarrhythmic drugs, cardiotonic drugs and other cardiovascular system drugs. In recent years, the number of adverse reactions/events reported and the proportion of serious reports in the cardiovascular system have shown an upward trend, suggesting that more attention should be paid to the risks of such drugs.
In 2021, the National Adverse Drug Reaction Monitoring Network received a total of 186,000 adverse reactions/events reported on drugs used in the cardiovascular system, accounting for 9.5% of the overall reports; of which 11,129 were severe reports, accounting for 6.0%.
2.1 Situations involving drugs
In 2021, the top 3 drug categories in the number of adverse reactions/events reported on the use of drugs in the cardiovascular system were blood pressure lowering drugs, antianginal drugs, and anti-atherosclerotic drugs; the top 3 drug categories in the number of serious reports of drugs in the cardiovascular system were anti-atherosclerotic drugs, blood pressure lowering drugs, and antianginal drugs.
In the 2021 cardiovascular system adverse drug reactions/event reports, injections accounted for 29.4%, oral formulations accounted for 69.3%, and other dosage forms accounted for 1.3%; in serious reports, injections accounted for 40.2%, oral preparations accounted for 58.5%, and other dosage forms accounted for 1.3%.
2.2 Organ system involvement
In the 2021 Cardiovascular System Adverse Reactions/Events Report, the top 5 organ system-related oral formulations were various neurological diseases, gastrointestinal system diseases, systemic diseases and various reactions at the site of administration, respiratory, thoracic and mediastinal diseases, skin and subcutaneous tissue diseases; the top 5 injectable organ system diseases were various nervous system diseases, gastrointestinal system diseases, skin and subcutaneous tissue diseases, systemic diseases and various reactions at the site of administration, and heart organ diseases (Figure 8).
Figure 8 Adverse drug reactions/events involving organ systems in 2021
2.3 Monitoring situation analysis and safety risk tips
In the 2021 cardiovascular system adverse drug reactions/events report, the proportion of oral preparations reported was significantly higher than that of injections, suggesting that the report of adverse drug reactions/events in the cardiovascular system came more from the oral administration route. In the report of serious adverse reactions/events, atorvastatin and rosuvastatin, which are reported in the top two, are statins, and lipid modulating drugs are used not only for the treatment of dyslipidemia and related cardiovascular diseases, but also for the prevention of such diseases. In addition, it is not excluded that there are cases caused by unreasonable, irregular use and drug interactions, suggesting that medical staff and patients should pay attention to the risks of such drugs.
3. Monitoring of adverse drug reactions in metabolic and endocrine systems
Drugs for the metabolic and endocrine system refer to drugs that treat endocrine and metabolic-related diseases, including glucocorticoids, diabetes treatment drugs, anti-gout drugs, thyroid disease drugs, pituitary gland disease drugs, etc. In recent years, the number of adverse reactions/events reported and the proportion of severe reports of adverse reactions/events in the metabolic and endocrine systems have shown an upward trend, suggesting that more attention should be paid to the risks of such drugs.
In 2021, the National Adverse Drug Reaction Monitoring Network received a total of 81,000 reports of adverse drug reactions/events in the metabolic and endocrine systems, of which 7,422 were severe reports, accounting for 9.2%. Reports of adverse drug reactions/events in the metabolic and endocrine systems accounted for 4.1% of the overall reports in 2021.
3.1 Situations involving drugs
In 2021, the top 3 drug categories in terms of reported number of adverse reactions/events in the metabolic and endocrine system were glucocorticoids, biguanides, and other diabetes treatment drugs (except insulin, insulin secretory drugs, biguanides, α-glycosidase inhibitors, and other diabetes treatment drugs other than thiazolidinediones, the same below), and the top 3 drug categories in terms of the number of serious adverse reactions/events reported were glucocorticoids, insulin, and antithyroid drugs.
In the 2021 metabolic and endocrine system adverse drug reactions/events reports, oral preparations accounted for 56.6%, injections accounted for 37.2%, and other dosage forms accounted for 6.2%. Serious adverse reactions/event reports accounted for 50.7% of oral formulations, 42.0% of injections and 7.3% of other dosage forms.
3.2 Organ system involvement
In the 2021 Metabolic and Endocrine System Adverse Drug Reactions/Events Report, the overall reported and severely reported adverse drug reactions/events involving the organ system are detailed in Figure 9. Compared with the overall report of drugs for the metabolic and endocrine systems, the proportion of severely reported adverse drug reactions/events involving organ systems, metabolic and nutritional diseases, various neurological diseases, various examinations, respiratory, thoracic and mediastinal diseases, and cardiac organ diseases is significantly higher.
Figure 9 Adverse reactions/events of metabolic and endocrine system drug use affect organ systems in 2021
In the overall adverse reactions/event reports of metabolic and endocrine system medications, the top 5 organ system diseases involving oral preparations are gastrointestinal system diseases, skin and subcutaneous tissue diseases, metabolic and nutritional diseases, various nervous system diseases and systemic diseases and various reactions at the site of administration; the top 5 injection involvement organ system diseases are gastrointestinal system diseases, skin and subcutaneous tissue diseases, metabolic and nutritional diseases, systemic diseases and various reactions at the site of administration, and various nervous system diseases.
In the report on serious adverse drug reactions/events of metabolic and endocrine system medications, the top 5 organ system diseases of oral preparations are gastrointestinal system diseases, metabolic and nutritional diseases, skin and subcutaneous tissue diseases, various neurological diseases, and various examinations; the top 5 of injection involvement organ systems are metabolic and nutritional diseases, gastrointestinal system diseases, various nervous system diseases, skin and subcutaneous tissue diseases, and various examinations.
3.3 Monitoring situation analysis and safety risk tips
In terms of the absolute number of reports, the number of reported diabetes treatment drugs has increased the most compared with 2020; from the ranking of the total reports and the number of severe reports of various varieties, some of the newer diabetes treatment drugs (such as polyethylene glycol lotherna peptide, dulaglucide, dapagliflozin) have risen rapidly. On the one hand, this may reflect the increase in the incidence and/or diagnosis rate of diabetes due to the aging of the mainland population and the improvement of medical security, which may lead to the expansion of the use of diabetes treatment drugs, and on the other hand, it also suggests that prescribers and patients should pay attention to the associated risks when choosing diabetes treatment drugs, especially newer drugs.
4. Monitoring of adverse reactions of injections
The overall number of reported adverse reactions/events with injectables (excluding vaccines) in 2021 increased by 14.7% compared with the same period in 2020, and the proportion of overall drug reports was basically the same as the overall situation in recent years. According to dosage form statistics, 55.5% of the total adverse reactions/events reported by drugs in 2021 were injectable (without vaccines) and 70.9% were reported in severe reports.00% were injectable (without vaccines). According to the statistics of drug classification, chemical injections accounted for 87.8%, Chinese medicine injections accounted for 6.4%, biological products accounted for 3.1%, and unclassifiable people accounted for 2.7%; injections (excluding vaccines) serious reports chemical drug injections accounted for 87.4%, Chinese medicine injections accounted for 4.7%, biological products accounted for 5.7%, and unclassifiable people accounted for 2.2%.
4.1 Drug situation
The top 3 drug categories in terms of chemical injection reports are anti-infectives, oncology drugs, electrolytes, acid-base balance, and nutritional drugs (Figure 10).
Figure 10 Adverse reactions/incident reports of chemical injections in 2021 cover the drug category
The top 5 in the overall report category of Chinese medicine injections are blood care agents, tonics, openers, antipyretic agents, and expectorants (Figure 11).
Figure 11 Adverse reactions/events reported on Chinese medicine injections in 2021 relate to the drug category
4.2 Organ system involvement
In the 2021 injection overall adverse reactions/events report, the top 5 organ system involvement are skin and subcutaneous tissue diseases, gastrointestinal system diseases, systemic diseases and various reactions at the site of administration, various neurological diseases and various examinations. Among the serious adverse reactions/events of injections, the top 5 diseases involving the organ system are blood and lymphatic diseases, various examinations, skin and subcutaneous tissue diseases, systemic diseases and various reactions at the site of administration, and gastrointestinal system diseases (Figure 12).
Figure 12 Adverse reactions/events of injections affecting organ systems in 2021
4.3 Monitoring situation analysis and safety risk tips
From the perspective of dosage form statistics, the overall number of reported adverse reactions/events of injections (excluding vaccines) in 2021 increased by 14.7% compared with the same period in 2020, and the proportion of overall drug reports was basically the same as that of recent years. From the statistics of the drug population, the number of reported adverse reactions/events of injectables (excluding vaccines) in children increased by 19.7% compared with the same period in 2020, and the overall proportion was basically the same as the overall situation in recent years. According to the monitoring of injections, it is recommended that clinicians carefully read the product instructions before taking drugs, pay close attention to relevant safety content, conduct sufficient benefit and risk analysis before prescription, and always follow the principle of "can take medicine without injection, can inject without infusion" to rationally choose medication. As a special drug population, children are affected by factors such as incomplete organ development, are more sensitive to drugs, have poor tolerance, and should be used with caution.
More pharmacovigilance
Follow the Clinical Pharmacy Channel to view
Source: Clinical Pharmacy Channel of the Medical Community
Editor-in-charge: Zheng Huaju
Proofreader: Zang Hengjia
Plate making: Xue Jiao