People's Daily health client Liu Meiyan
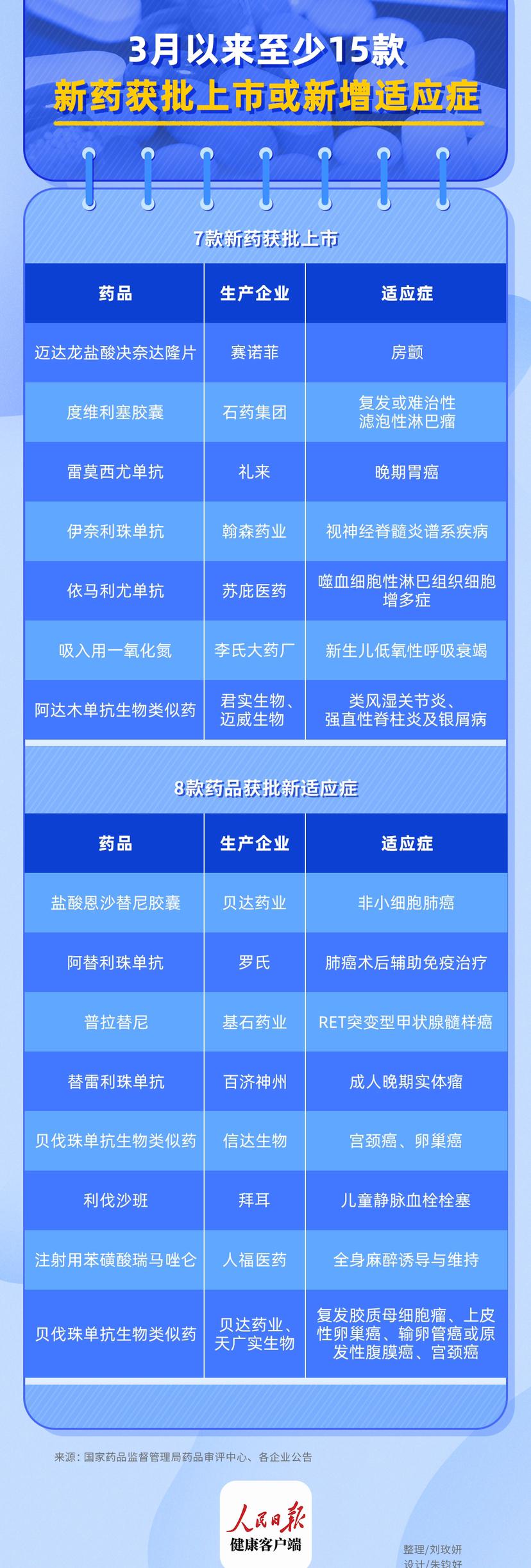
Since March, the mainland has ushered in a number of new drugs on the market, according to the people's daily health client incomplete statistics, at least 7 new drugs have been approved for listing, 8 drugs have been approved for new indications.
Medicines: Midalone dunedalone hydrochloride tablets
Manufacturer: Sanofi
Indications: Atrial fibrillation
On March 19, Sanofi China announced the launch of its anti-arrhythmic drug, Midalone Dunadalone Hydrochloride Tablets, in China for sinus rhythm patients with a history of paroxysmal or persistent atrial fibrillation (atrial fibrillation), reducing the risk of hospitalization for atrial fibrillation.
Medicines: dovelise capsules
Manufacturer: CSPC Pharmaceutical Group
Indications: Recurrent or refractory follicular lymphoma
On March 18, CSPC's new anti-cancer drug Duvelisa capsules introduced by CSPC group was approved in China for the treatment of adult patients with relapsed or refractory follicular lymphoma who had undergone at least two systematic treatments in the past. This is a dual inhibitor of PI3K-δ and PI3K-γ, and CSPC Pharmaceutical Group has the exclusive license to develop and commercialize the product in Greater China.
Medicines: ramoxizumab
Manufacturer: Eli Lilly
Indications: Advanced gastric cancer
On 18 March, Eli Lilly's VEGFR-2 monoclonal antibody remosilumab injection was approved for marketing in China for the second-line treatment of advanced gastric cancer, that is, in combination with paclitaxel for the treatment of patients with advanced gastric or gastroesophageal-conjugate adenocarcinoma with disease progression during or after chemotherapy- This is also the first and only targeted drug approved for the second-line treatment of advanced gastric cancer in China.
Medicines: Inelizumab
Manufacturer: Hansen Pharmaceutical
Indications: Neuromyelitis optic disease
On March 14, the anti-CD19 antibody inelizumab introduced by Hansen Pharmaceutical was approved for marketing in China. In the United States, the drug has been approved for the treatment of patients with optic neuromyelitis spectrum disease (NMOSD). NMOSD is a rare, severe autoimmune disease that attacks the optic nerve, spinal cord, and brainstem, causing blindness and paralysis.
Medicines: emmaliumab
Manufacturer: Sophie Pharmaceuticals
Indications: Hemophagocytic lymphocytosis
On 11 March, Subi's interferon γ (IFNγ) antibody emaliumab injection was approved in China for the treatment of adults and children with refractory, recurrent or progressive disease or primary hemophagocytosis (HLH) who are intolerant to conventional HLH therapy.
Medicines: Nitric oxide for inhalation
Manufacturer: Lee's Pharmaceutical Factory
Indications: Neonatal hypoxic respiratory failure
On 11 March, the vasodilator inhaled nitric oxide introduced by Lee's Pharmaceuticals was approved for marketing in China in combination with ventilation support and other appropriate drugs for the treatment of hypoxic respiratory failure (HRF) in newborns with pulmonary hypertension as evidenced by clinical or echocardiography, thereby improving oxygenation function and reducing the need for extracorporeal membrane oxygenation (ECMO).
Medicines: adalimumab biosimilars
Production enterprises: Junshi Biological, Maiwei Biological
Indications: Rheumatoid arthritis, ankylosing spondylitis and psoriasis
On March 3, the biosimilar adalimumab developed by Junshi Biotech in collaboration with Maiwei Bio was approved for marketing in China for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriasis. Adalimumab, originally developed by AbbVie, is a monoclonal antibody of TNF-α, which was first listed in the United States in 2002 and entered the Chinese market in 2010, with the trade name "Humira".
In addition, 8 drugs were approved for new indications in March:
Medicines: ensartinib hydrochloride capsules
Manufacturer: Beida Pharmaceutical
New indication: Non-small cell lung cancer
Beida Pharmaceuticals' ensartinib hydrochloride capsules were approved as new indications in China. This is a highly selective next-generation anaplastic lymphoma kinase (ALK) inhibitor approved for first-line treatment in patients with ALK-positive locally advanced or metastatic non-small cell lung cancer (NSCLC).
Medicines: atenizumab
Manufacturer: Roche
New indications: Adjuvant immunotherapy after lung cancer
Roche's PD-L1 inhibitor atenizumab has been approved as a new indication in China for the detection of adjuvant therapy in patients with stage II-IIIA NSCLC who are evaluated as ≥1% tumor cell (TC) PD-L1 stain positive, surgically resected, and platinum-based chemotherapy. As of the day of approval, this is the first and only approved postoperative adjuvant immunotherapy indication for non-small cell lung cancer in China, which is expected to further reduce the risk of disease recurrence in patients with postoperative lung cancer.
Medicines: pratinib
Manufacturer: CStone Pharmaceuticals
New indication: RET mutant medullary thyroid carcinoma
Pratinib, a RET inhibitor introduced by CStone, has been approved as a new indication in China for the treatment of adults and children aged 12 years and older with advanced or metastatic RET mutant medullary thyroid cancer requiring systemic therapy, as well as advanced or metastatic RET fusion-positive thyroid cancer and children aged 12 years and older who require systemic therapy and refractory radioactive iodine.
Medicines: terelizumab
Manufacturer: BeiGene
New indications: advanced solid tumors in adults
BeiGene anti-PD-1 monoclonal antibody terelizumab was approved as the seventh indication in China for the treatment of adult patients with advanced solid tumors with unresectable or metastatic microsatellites of highly unstable or mismatched repair of genetic defects.
Medicines: biosimilars of bevacizumab
Manufacturer: Cinda Biologics
New indications: Cervical cancer, ovarian cancer
Innovent Bio bevacizumab biosimilars were approved in China for the 5th and 6th indications, namely: combination of carboplatin and paclitaxel for the first-line treatment of patients with stage III or IV epithelial ovarian, fallopian tube cancer or primary peritoneal cancer after initial surgical resection, and paclitaxel and cisplatin or paclitaxel and topotecan for the treatment of patients with persistent, recurrent or metastatic cervical cancer.
Medicine: Rivaroxaban
Manufacturer: Bayer
New indication: Venous thromboembolism in children
Bayer's factor Xa anticoagulant rivaroxaban has been approved as a new indication in China for VTE treatment and prevention of VTE recurrence in children and adolescents under 18 years of age weighing 30 kg-50 kg and over 50 kg of venous thromboembolism (VTE) at least 5 days after initial non-oral anticoagulation. Previously, the drug has been approved in China for the prevention and treatment of adult venous thrombosis, chronic coronary artery disease or peripheral artery disease patients after hip/knee artery replacement and other indications.
Medicines: remazolam besylate for injection
Manufacturer: Renfu Pharmaceutical
New indications: induction and maintenance of general anesthesia
Yichang Renfu Pharmaceutical, a holding subsidiary of Renfu Pharmaceutical, and The German company PAVON jointly developed ramazolam for injection benzesulfonate has been approved as a new indication in China. The new indication is "induction and maintenance of general anesthesia". The drug has previously been approved in China for colonoscopy sedation.
Manufacturers: Beida Pharmaceutical, Tianguangshi Biology
New indications: recurrent glioblastoma, epithelial ovarian cancer, fallopian tube carcinoma or primary peritoneal cancer, cervical cancer
The bevacizumab biosimilar co-developed by Beida Pharmaceutical and Tianguangshi Biotech has been approved for four new indications in China, namely recurrent glioblastoma, epithelial ovarian cancer, fallopian tube carcinoma or primary peritoneal cancer, and cervical cancer.