People's Daily Health Client Reporter Gao Ruirui
On January 18, the National Health Insurance Administration, together with the Ministry of Human Resources and Social Security and other departments, released the National Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance Drug Catalogue (2022). According to the incomplete combing of the People's Daily health client, many of the new drugs in the medical insurance catalogue were approved for the first time in China in the first half of 2022 and entered the catalogue through negotiation, involving 10 new drugs such as roprostim for injection, rotecept for injection, and upatinib sustained-release tablets.
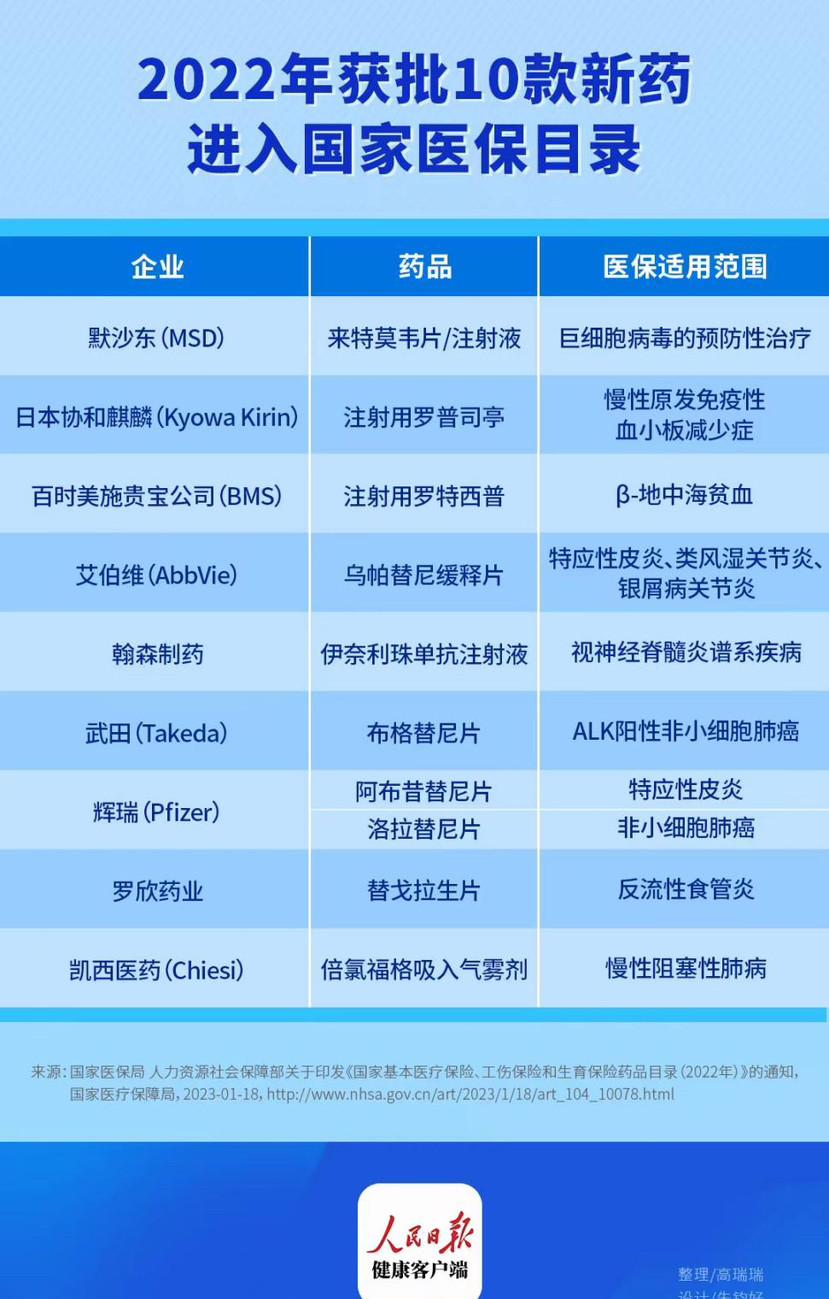
Merck (MSD): Letemovir tablets/injection
Scope of medical insurance: preventive treatment of cytomegalovirus
Letemovir is a novel non-nucleoside cytomegalovirus (CMV) inhibitor that exerts antiviral effects by inhibiting the activity of CMV terminal enzyme complexes and preventing the processing and packaging of viral DNA.
According to Merck's (MSD) official website, letemovir tablets and letemovir injection were approved in China in December 2021 and May 2022, respectively, for the prevention of cytomegalovirus infection and cytomegalovirus disease in adult recipients who receive allogeneic HSCT seropositive [R+]. This time, these two dosage forms approved for letermovir in China have been included in the medical insurance catalog.
Merck Sharp & Dohme official website photo
Ropustim is a second-generation TPO receptor agonist that stimulates intracellular transcriptional pathways by binding to TPO receptors, resulting in increased platelet production. The drug was selected as "Best Biotechnology Product" by the Galen Award, known as the "Nobel Prize in the pharmaceutical industry", in 2009.
Ropustine was approved for marketing in China in January 2022, and the scope of application of this inclusion in the medical insurance catalogue is limited to adult (≥ 18 years old) patients with chronic primary immune thrombocytopenia (ITP) who do not respond well to other treatments (e.g., corticosteroids, immunoglobulins).
Bristol-Myers Squibb (BMS): Rotecept for injection
Medical insurance coverage: β-thalassemia
Rotercept is a red blood cell maturation agent. It is formed by fusing the Fc domain of human immunoglobulin G1 (IgG1) with the extracellular domain of the activated hormone receptor IIB (ActRIIB), which regulates advanced erythrocyte maturation and improves ineffective hematopoiesis.
In January 2022, roctecept was approved for marketing in China for the treatment of adult patients with β-thalassemia who require regular red blood cell transfusions and red blood cell transfusions ≤ 15 units for 6/24 weeks. The scope of application of the drug included in the medical insurance catalogue is: limited to adult patients with β-thalassemia.
AbbVie: Upatinib sustained-release tablets
Medical insurance coverage: atopic dermatitis, rheumatoid arthritis, psoriatic arthritis
Upatinib is a once-daily, selective and reversible JAK1 inhibitor that has shown significantly greater inhibitory efficacy against JAK2, JAK3 and TYK2.
The drug was approved for three adaptations in China in the first half of 2022, and this time they were all included in the scope of application of the medical insurance catalog, specifically: second-line treatment of refractory, moderately severe atopic dermatitis in patients aged 12 years and above; second-line therapy in adult patients with active psoriatic arthritis; Second-line treatment for adults with moderate to severe active rheumatoid arthritis.
Hansen Pharmaceutical: Inelizumab injection
Medical insurance coverage: neuromyelitis optica spectrum diseases
Inelizumab is an anti-CD19 monoclonal antibody that consistently reduces neuromyelitis optica spectrum disease (NMOSD) recurrence by targeting CD19+ B cells for wider and longer depletion of B cells. NMOSD is a rare autoimmune disease of the central nervous system, and most patients produce autoantibodies to a waterporin called AQP4 in the body.
Inelizumab was approved in China in March 2022, and the scope of application for inclusion in the medical insurance catalogue this time is: limited to adult patients with NMOSD who are positive for anti-AQP4 antibody.
Takeda: Burkina tablets
Medical insurance coverage: ALK-positive non-small cell lung cancer
Buritinib tablets are a new generation of tyrosine kinase inhibitors. It is designed to specifically target and inhibit anaplastic lymphoma kinase (ALK) fusion protein, which can inhibit ALK and ALK fusion protein, thereby inhibiting tumor growth.
Burglitinib was approved in China in March 2022, and this time it is included in the medical insurance catalogue for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who are limited to ALK-positive.
Pfizer: Albuxitinib tablets
Scope of medical insurance: atopic dermatitis
Albuxitinib tablets are a once-daily oral highly selective JAK1 inhibitor. JAK1 inhibits a variety of cytokines thought to regulate the pathophysiology of atopic dermatitis.
Albuxitinib tablets were approved for marketing in China in April 2022, and the scope of application of this inclusion in the medical insurance catalogue is: limited to adult patients with refractory, moderately to severe atopic dermatitis who do not respond well to other systemic therapies (such as hormones or biological agents) or are not suitable for the above treatment.
Luoxin Pharmaceutical: Tegolaxen tablets
Scope of medical insurance: reflux esophagitis
Tegolaxen tablets are potassium competitive acid blockers (P-CAB), which can block the potassium channel of H+/K+-ATPase, competitively block the binding of potassium ions to the enzyme, stay in the stomach parietal cells for a long time, thereby rapidly inhibiting the secretion of gastric acid.
Tegolaxen tablets were approved in China in April 2022, and the scope of application of this inclusion in the medical insurance catalogue is: reflux esophagitis.
Chiesi: Beclovogel inhalation aerosol
Scope of medical insurance: chronic obstructive pulmonary disease
According to the public information of Case Pharma, beclovogel inhalation aerosol is an inhaled glucocorticoid (ICS)/long-acting muscarinic receptor antagonist (LAMA)/long-acting β2-adrenoceptor agonist (LABA) triple combination inhalation preparation, the active ingredients of which are beclomethasone propionate, formoterol fumarate and glycopyrronium bromide. Studies have shown that triple therapy with ICS/LAMA/LAMA improves lung function and reduces the risk of exacerbations in people with chronic obstructive pulmonary disease (COPD) better than monotherapy or dual therapy. In addition, the product has a unique ultra-fine anti-fog technology that improves small airway function.
In April 2022, Beclovogel inhalation aerosol was approved in China for maintenance therapy in patients with COPD. The scope of application of the drug included in the medical insurance catalogue is: limited to chronic obstructive pulmonary disease.
Pfizer: Lolatinib tablets mechanism of action: ALK inhibitor
Medical insurance coverage: Non-small cell lung cancer
Lolatinib is a third-generation ALK inhibitor developed by Pfizer. It inhibits mutations in the ALK gene that are resistant to other ALK inhibitors and can cross the blood-brain barrier to treat brain metastases. According to the clinical data presented by the researchers at the 2022 American Association for Cancer Research (AACR) annual meeting, the progression-free survival of ololatinib in the first-line treatment of ALK-positive non-small cell lung cancer (NSCLC) has exceeded 3 years; The objective intracranial response rate of patients with brain metastases after treatment with this product was as high as 83.3%.
Lolatinib tablets were approved for marketing in China in April 2022, and the scope of application for inclusion in the medical insurance catalogue is: limited to ALK-positive patients with locally advanced or metastatic NSCLC.