The Year of the Tiger has arrived, and China's pharmaceutical industry has ushered in a new year.
The national ice and snow sports triggered by the Winter Olympics and the collection of the pharmaceutical industry have undergone a fantastic linkage, and the Beijing-Tianjin-Hebei "3+N" Alliance has released an orthopedic collection plan to escort the injured including ski injuries.
Regarding the collection, the State Medical Insurance Bureau, the Food and Drug Administration, the Health Commission, and the Ministry of Industry and Information Technology jointly introduced the reform progress of "drug and high-value medical consumables collection" at the briefing of the state council. Provincial alliance collection is clearly defined as the mainstream trend of future collection and mining.
On the side of pharmaceutical companies, the news that Cinda PD-1 has been blocked from going to sea has aroused the attention and discussion of the whole industry; the further announcement of the details of the AstraZeneca fraud case has also warned all pharmaceutical companies; the release of the 2021 financial reports of major multinational pharmaceutical companies shows that the industry is always in change and competition, showing a thriving posture.
The Health Bureau has compiled more hot information this week as follows:
Blockbuster policy
1. The use of collected drugs is included in the assessment of public hospitals
On February 11, the new office of the State Council held a regular policy briefing to introduce the centralized procurement of drugs and high-value medical consumables. Wang Xuetao, head of the Department of Pharmaceutical Policy of the National Health Commission, said that the collection of drugs and consumables has entered the stage of normalization and institutionalization. The National Health Commission has formulated and revised some guidelines for clinical drugs, further promoted the preferential and rational use of collected drugs and consumables, and better served the health needs of the people.
2. Multiple provinces issued DRG/DIP reform plans
Recently, Shaanxi, Fujian, Anhui, Liaoning, Inner Mongolia and other provinces have intensively issued a three-year action plan for dreg/DIP payment method reform to further clarify the implementation of medical insurance payment method reform.
According to the overall action plan of the National Medical Insurance Bureau, by the end of 2024, all co-ordinating areas in the country will carry out the reform of DRG/DIP payment methods, and the pilot areas will be launched in the early stage to continuously consolidate the reform results. By the end of 2025, the DRG/DIP payment method will cover all eligible medical institutions that carry out inpatient services, and basically achieve full coverage of disease types and medical insurance funds.
In 2022, the reform of DRG/DIP payment methods will be officially rolled out from local pilots to the whole country, and the new medical insurance payment methods will put forward higher requirements for the medical equipment and consumables industries. Under the premise of ensuring the effectiveness of diagnosis and treatment, the consideration of cost-effectiveness will be placed in a more important position by the hospital. In the DRG/DIP reform, the cost-effective advantage of domestic equipment will be more obvious.
3. The Medical Insurance Bureau publishes a number of key monitoring catalogues
On February 8, the Fujian Provincial Pharmaceutical and Equipment Joint Procurement Center issued the Notice on Announcing the Third Batch of Medical Insurance Key Monitoring Drugs and the List of Key Drugs, requiring relevant units to strengthen key monitoring and focus on drug management.
The third batch of medical insurance key monitoring drug list involves a total of 30 varieties, of which the third batch of medical insurance key monitoring drugs are 9, including Buchang Pharmaceutical's Brain Heart Capsule and AstraZeneca's Omeprazole sodium for injection; the third batch of medical insurance focuses on 21 drugs, including Tasly's compound danshen drop pills, stone medicine Enbipro's butylphthalide soft capsules, Bayer's Rivaroxaban tablets.
Industry events
1. Cinda PD-1 was blocked from going to sea
In the early morning of February 11, the FDA held a meeting of the Advisory Committee on Oncology Drugs (ODAC) to discuss the U.S. marketing application for the Cinda PD-1 inhibitor sindilimab. The expert committee voted 14:1, concluding that sindilizumab should be supplemented with clinical trials to demonstrate the applicability of the drug in the U.S. population and in U.S. medical practice before it could be approved.
The Health Bureau learned that the rejection of sindilizumab by the FDA focused on three points, the core of which is that the FDA believes that the clinical research of Cinda is not an international multi-center clinical study, on the one hand, the authenticity of the results cannot be guaranteed, on the other hand, whether the clinical research data of a single country is applicable to patients in the United States is not clear. In addition, the endpoints of clinical studies are also controversial points.
This is the first time that China's local pharmaceutical companies have talked with regulators through the ODAC meeting, and the overseas participation of xindilizumab has attracted much attention in the industry, and its obstruction also shows to a certain extent that the difficulty of China's anti-cancer drugs going to sea is increasing.
In this regard, Cinda and Eli Lilly responded that they regretted the voting results, but would still cooperate with the FDA to continue to complete the review of the new drug declaration. According to a number of media reports, Eli Lilly originally hoped that through the letter dilizumab, it could affect the US health care system through an aggressive price strategy.
2. A number of multinational pharmaceutical companies announced their 2021 results
At the beginning of February, multinational pharmaceutical companies announced their 2021 results. Johnson & Johnson, Pfizer, Roche, AbbVie, Novartis, Merck, Bristol-Myers Squibb, GlaxoSmithKline, Sanofi, and AstraZeneca make up the top 10 total revenue rankings.
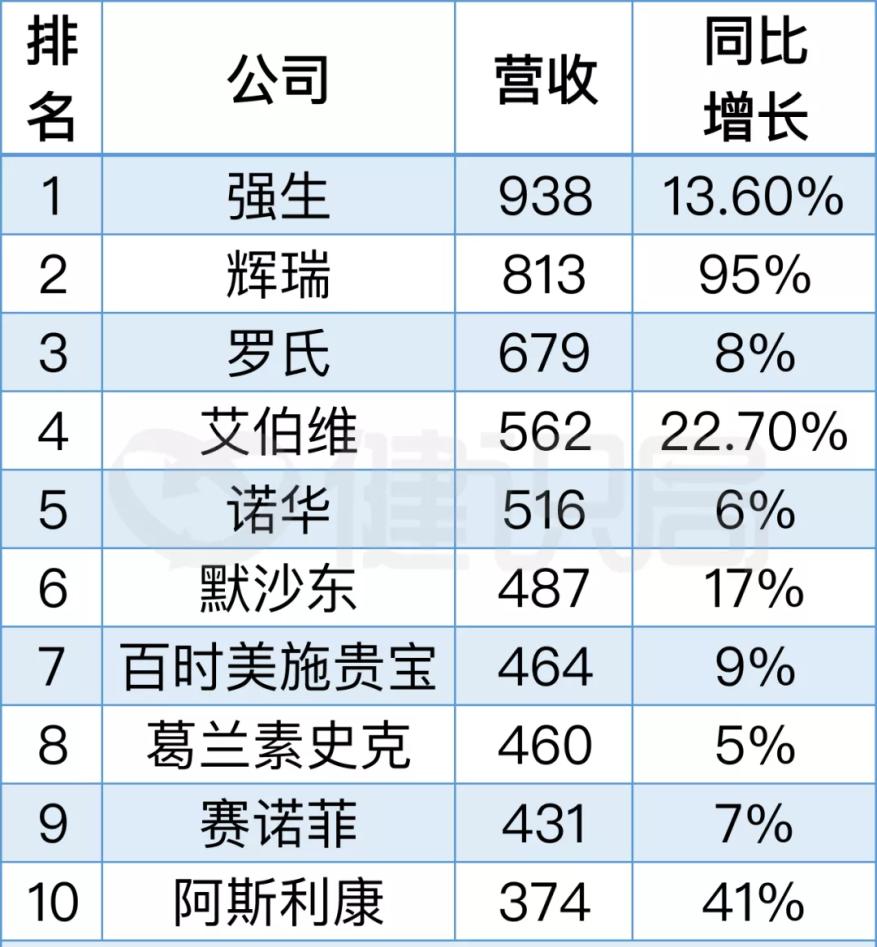
Pfizer's revenue increased by 95% year-on-year, and the annual sales of covid-19 vaccines reached $36.8 billion, showing a strong trend among multinational pharmaceutical companies in addition to Johnson & Johnson. AstraZeneca's revenue growth rate of 41% is also quite impressive, replacing Takeda and squeezing into the top ten, but in the Chinese market, revenue has rarely declined.
3. GSK executives join Bayer
Recently, the Health Bureau learned that Christine Roth, senior vice president of GSK's global oncology business, will join Bayer on March 1 as the head of the oncology business, reporting to Stefan Oelrich, member of the Bayer Group Management Committee and global president of the prescription drug division. Robert LaCaze, the former head of the department, decided to leave Bayer to pursue his own career and go to the unknown.
Christine Roth has served as an executive at GlaxoSmithKline, Novartis and Bristol-Myers Squibb, focusing on global product strategy and commercialization, building oncology pipelines and driving new product launches.
4. Teva will pay $3.6 billion to settle the lawsuit
On Feb. 9, Teva, the world's largest generic drug company, said it was prepared to pay up to $3.6 billion in cash and drugs to close thousands of lawsuits alleging that Teva and other pharmaceutical companies had exacerbated the opioid epidemic in the United States.
Opioid addiction has become an epidemic in the United States. According to the U.S. Centers for Disease Control and Prevention (CDC), between 1999 and 2017, the number of deaths from prescription opioid overdose in the United States was close to 250,000. In 2017, the U.S. Department of Health and Human Services officially confirmed the crisis and declared a public health emergency. Over the years, at least six pharmaceutical companies, including Johnson & Johnson and Teva, have faced allegations of deliberately violating the Controlled Substances Act for opioids for non-medical purposes.
New drugs are approved
1. Pfizer's new crown oral drug was approved to enter China
On the morning of February 11, the State Food and Drug Administration conditionally approved the import registration of Pfizer's new coronavirus treatment drug nematvir tablets/ritonavir tablets combination packaging (i.e. Paxlovid).
Paxlovid is an oral small molecule covid-19 treatment for adults with mild to moderate COVID-19 (COVID-19) with a high risk factor for progression to severe illness. The State Food and Drug Administration requires the marketing authorization holder to continue to carry out relevant research work, complete the conditional requirements within a time limit, and submit the follow-up research results in a timely manner.
It is understood that Pfizer is accelerating the increase in production capacity, and Gloria Ying and Tempo shares are also predicted by the market to be the service providers of Pfizer's new crown oral drugs.
2. Five anti-ED drugs were removed from the shelves
Recently, anti-ED drugs sold online by overseas retailers such as Amazon and Walmart have been found to be suspected of adulterating tadalafil ingredients and have been removed from the shelves and recalled.
Tadalafil is an FDA-approved phosphodiesterase inhibitor for erectile dysfunction in men, and U.S. regulatory authorities do not allow any patient to use it without a doctor's prescription. The five drugs recalled include The Red Pill, Mac Daddy Red, Mac Daddy Purple, Red Mammoth and MegMan performance booster.
3. The first lupus nephritis drug was approved in China
On 10 February, GlaxoSmithKline announced that bailiyumab for injection has been approved by the National Food and Drug Administration for use in adult patients with active lupus nephritis (LN) in combination with conventional treatment.
In July 2019, the drug was approved by the State Food and Drug Administration for the treatment of systemic lupus erythematosus, which was approved for the treatment of lupus nephritis, expanding the indications. This is the first and only biologic agent in China to cover indications for systemic lupus erythematosus and lupus nephritis.
4. Sinopharm's new crown vaccine is listed in South Africa
On February 7, South Africa's Health Products Regulatory Authority announced that Sinopharm's COVID-19 vaccine has been approved for official use in the country for vaccination of people aged 18 years and older
In order to gain market access, Sinopharm has partnered with a local South African company, MC Pharma Pty (Ltd), which began submitting rolling applications for the vaccine as early as July 2021. South Africa is the country with the worst COVID-19 outbreak in Africa. Previously, the COVID-19 vaccine produced by China's Kexing was approved for emergency use in South Africa in July 2021.
5. Watson Bio's covid-19 vaccine for mutant strains has been approved for clinical trials abroad
On February 9, Watson Biotech announced that the recombinant novel coronavirus variant vaccine (CHO cells) independently developed by its holding subsidiary, Shanghai Zerun, was approved by the Ethics Committee of The Barmako University of Science and Technology in Mali, and agreed to carry out Phase I/II clinical trials of the vaccine in Mali to evaluate the safety, reaction and immunogenicity of the vaccine in healthy adults aged 18 to 60 years old.
Previously, Shanghai Zerun already had a vaccine against the prototype strain of the new coronavirus, which was in the phase II clinical trial stage. The vaccine approved for clinical trials is a vaccine against the novel coronavirus variant.
#Pfizer ##集采 #