Soft tissue sarcoma is a rare malignancy of mesenchymal sources, accounting for about 1% of adult cancers, although it is not common compared with lung cancer and breast cancer, but it is a complex tumor with strong heterogeneity, and there are more than 100 known subtypes, each with different clinical manifestations. Regardless of the disease, the clinical treatment options for advanced patients are very limited.
Eight years after the operation, the systemic metastases recurred, and new drugs allowed the tumor to subside in 7 days
In 2007, M, a boy who was just 14 years old, was diagnosed with a type of abdominal protrusive cutaneous fibrosarcoma, a type of soft-tissue sarcoma. Fortunately, the operation was very successful, and after the radical resection, there were no remaining lesions in the body, and he gradually returned to his health and returned to normal life. Everyone thinks cancer is completely gone.
In January 2015, eight years after his initial diagnosis, his tumor recurred, and the metastases in his chest were surgically removed again, followed by radiation therapy to remove the remaining cancer cells.
In 2016, it recurred again, and there were multiple metastases in the lungs and liver, this time unable to operate on, and only began to receive imatinib treatment, but unfortunately, the tumor continued to progress.
In 2017, because he did not want to suffer from chemotherapy anymore, Mr. M gave up all treatment and went home to recuperate.
In 2019, Mr. M, 29, went to the hospital for treatment for a fracture caused by bone metastasis throughout his body, and this examination made him completely desperate. PET shows multiple bone metastases in the hip bone, bilateral ribs, and left femur, multiple lesions in both lungs, multiple liver metastases, muscle metastases, and 1 suspected metastase in the left kidney. The tumor had completely engulfed him.
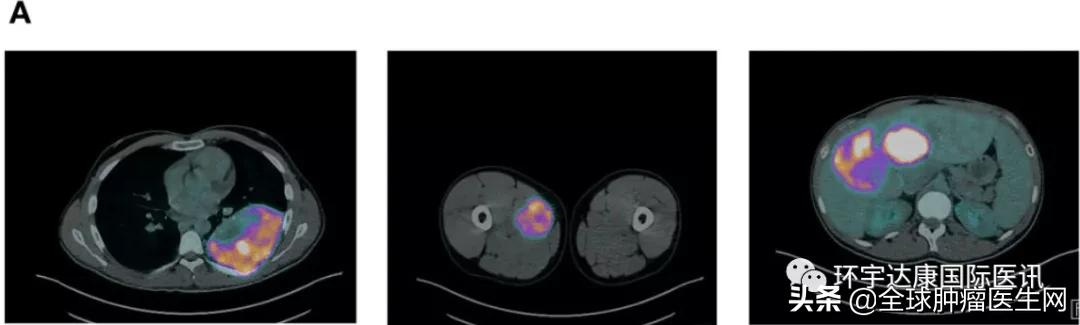
Just when Mr. M thought that his short life was coming to an end, his doctor did a whole gene test with his 2015 surgical tissue slices, and found a new mutation that saved his life - NTRK fusion (TPM4-NTRK1), a diamond target that has been popular in cancer circles in the past two years, and the two "curative" drugs for NTRK fusion, lalotinib and emtratinib, have also shocked the entire medical community.
In November 2019, Mr. M was fortunate enough to be admitted to the treatment of the famous lalotrectinib. In just 7 days, this amazing anti-cancer drug took effect. Pet results showed that tumors in the internal organs and bones were significantly reduced and that no significant side effects were observed. After continuing treatment for a period of time, Mr. M's tumors throughout his body showed a significant regression of fast reading, and until the time of the publication of the case in October 2021, Mr. M was still receiving treatment and responded well with no adverse events.
It has to be said that the development of precision medicine has allowed patients with extremely malignant tumors such as sarcoma to have a chance to survive for a long time!
Survive more than 2 years without progress! Lalotinib significantly prolongs survival in patients with sarcoma
Lalotinib can be counted as one of the biggest changes in the field of oncology in the past two years, which has made the dream of "broad-spectrum anti-cancer" shine into reality. The listing of lalotinib has saved thousands of unfortunate families, and at the same time, it has continuously refreshed its record in various tumors in international clinical research!
Recently, based on the results of three clinical trials (NCT02122913, NCR02576431 and NCT02637687), clinical data obtained by lalotinib in patients with refractory NTRK fusion-positive sarcoma were published, which once again caused a sensation.
Objective Response Rate (ORR):
A total of 25 adult sarcoma patients were included in these three studies, with an overall objective response rate (ORR) of 72% receiving ralotinib. thereinto:
In 19 patients with soft tissue sarcoma, the overall response rate reached 68%, including 3 complete responses (16%) and 10 partial responses (53%);
In addition, in 4 patients with gastrointestinal stromal tumors, the overall response rate reached 100%! including 1 complete response (25%) and 3 partial response (75%);
In 2 patients with osteosarcoma, the overall response rate was 50%.
Progression-free survival (PFS)
Overall, the median progression-free survival was 28.3 months for all patients and 52% for 24 months without progression.
Median survival (mOS)
At a median follow-up of 21.4 months, the median overall survival was 44.4 months and the 24-month survival rate was 91%. In patients with soft tissue sarcoma, the 24-month survival rate was 89%.
It is effective against 17 types of cancer and kicks off the prelude to "broad-spectrum anti-cancer"
As the world's first approved NTRK inhibitor, the "aura" on lalotinib is very dazzling. On November 26, 2018, the FDA expedited the approval of lalotinib for the treatment of adult and pediatric patients with locally advanced or metastatic solid tumors with NTRK fusion mutations who may lack standard treatment regimens or continue to progress after receiving other treatments.
With a high overall response rate, a high complete response rate, a long duration of remission, and a long duration of sustained patient benefit from treatment, lalotinib fully meets the patient's expectations for a targeted therapy drug, coupled with broad-spectrum anti-cancer properties, which is why it is called a "cure line" drug.
Clinical data have proven effective against 17 solid tumors, including 75% of common lung cancers and up to 100% thyroid cancer and fibrosarcoma! Of course, this data is still being refreshed in subsequent studies, and this drug does not distinguish between tumor sources, that is to say, no matter what refractory part of the tumor appears, whether there is metastasis or not, as long as there is NTRK (1/2/3) fusion, this drug can be used. It's finally here! The sky-high price of the "curative system" anti-cancer drug lalotinib has officially launched clinical trials in China!
Efficacy rate of various types of cancers in clinical trials of lalotinib
and duration of remission
note! Be sure to detect the target that unlocks the Key to Life, the NTRK
If lalotinib is a lock, the NTRK gene is the "key to life" that opens this lock, as long as there is NTRK fusion, it is equivalent to opening a door of hope.
However, this key is not available to every patient.
The first is because there are not many patients with NTRK gene fusion. For example, in lung cancer, breast cancer, and colorectal cancer common in China, only 1% to 5% of patients have this mutation, while some rare cancers, such as infant fibrosarcoma and secretory breast cancer, have NTRK fusion frequency of up to 90% to 99%.
Second, NTRK gene fusion is easily missed. The researchers found that even if you use a very good DNA-based NGS test, you can still miss some of these fusions. This is because the length of the introns detected is limited and it is difficult to find these fusions. The best way to detect this is to look for a assay that contains both DNA and RNA analysis in order to maximize the likelihood of detecting these fusions. This is not something that all domestic testing companies can do.
How are domestic patients treated?
At present, lalotinib has been listed in the United States, Japan, and Europe, but the annual cost of adults is 393,600 US dollars (nearly 2.6 million yuan), which is a sky-high price for domestic patients.
Fortunately, China's cancer patients have ushered in a good era, and in recent years, our country has greatly improved the speed of entering the market for new international anti-cancer drugs, and increased the intensity of independent research and development of new drugs. In addition to the upcoming market of lalotinib, there are two American NTRK inhibitors and five Chinese independent research and development of NTRK inhibitors officially approved in the country to carry out clinical trials, is recruiting patients, and there have been a large number of NTRK fusion patients through the Global Oncologist Network Medical Department successfully enrolled and received anti-cancer new drug treatment, successfully reversed the disease, we hope that more patients know this good news, more patients can benefit from these new anti-cancer drugs, defeat cancer!
As the oncology community gains more experience with these highly effective new drugs and genetic testing, physicians will become more adept at determining the most effective treatment for each patient.
In the future, under the guidance of science, rationally changing drugs and turning cancer into chronic diseases is our ultimate goal.