Everything in the universe is made up of atoms, and more than a hundred years ago, people once thought that atoms were the smallest particles that could be separated. But now we know that even tiny atoms have their own structure.
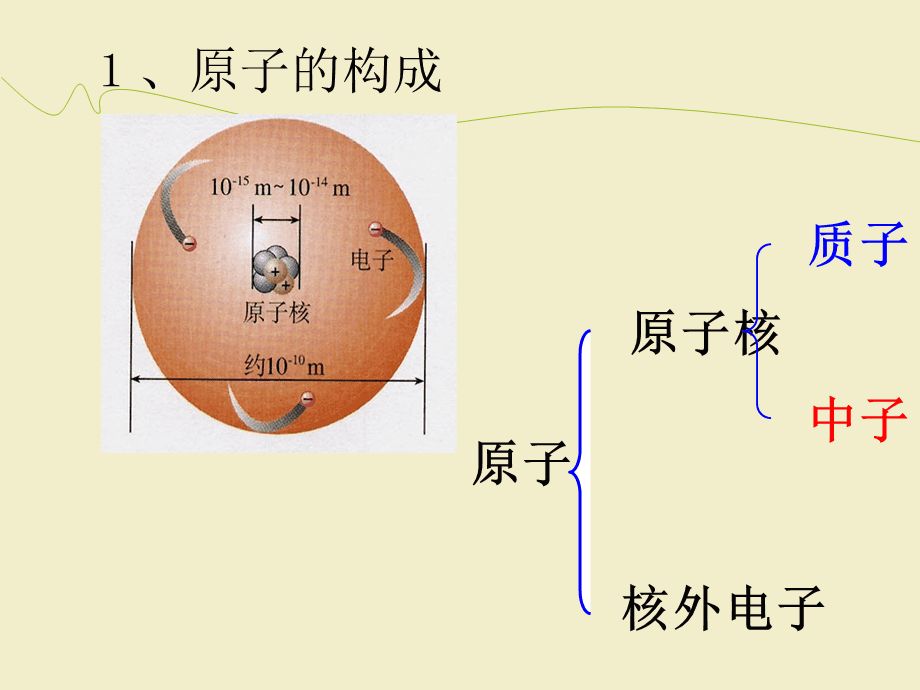
Atoms are made up of atomic nuclei and extranuclear electrons, and electrons are elementary particles. Protons and neutrons make up the nucleus, and both protons and neutrons are made up of quarks (three quarks), which are also elementary particles.
However, due to the existence of quark confinement, quarks cannot exist alone, and people cannot directly observe the existence of quarks.
So, how exactly is the internal structure of atoms distributed? A macro example can be seen intuitively.
Suppose you enlarge an atom to the size of a soccer field, how big are the nuclei and electrons?
The answer will surely surprise you. The nucleus is only the size of a bean and is located in the middle of the football field. And the electrons are even smaller, smaller than a speck of dust in the stands of a football stadium!
The nucleus and electron are so small, is the inside of the atom empty except for the nucleus and the electron?
It seems that the inside of the atom is really empty, but emptiness does not represent nothingness, and even on the contrary, it is very "active" there!
The nuclei are positively charged and the electrons are negatively charged. This means that there is a very strong electric field around the nucleus, and the electrons themselves have an electric field.
More importantly, electrons do not move in a fixed circle like the Earth around the sun, and electrons do not have a fixed orbit, but appear very randomly around the nucleus, like a large cloud, called an "electron cloud."
So, don't think that the inside of the atom is a vacuum, and don't think that objects can enter the inside of the atom at will. The existence of electron clouds looks like building layer after layer of "hard shells" around the nucleus, and ordinary particles are difficult to enter, unless uncharged particles like neutrinos can enter at will, and other particles are basically rejected.
This is also why the inside of the atom is theoretically very empty, but it is very dense and hard. Atoms are so hard that it is difficult for ordinary forces to compress them. To penetrate the "hard shell" of an atom's periphery, massive objects, such as the core region of a star, are needed, where the temperature and pressure are enormous.
Although the size of the nucleus is very small, it occupies more than 99.9% of the mass of the entire atom, and the density of the nucleus is very large, and the mass of a cubic centimeter of the nucleus is as high as 100 million tons!
The protons and neutrons that make up the nucleus of an atom are combined by powerful forces. What we usually call "the mass of protons plus neutrons equals the mass of the nucleus" is actually not rigorous, the former is smaller than the latter, because the combination of protons and neutrons occupies a part of the mass.
Further down, both protons and neutrons are made up of quarks, protons are made up of two upper quarks and one lower quark, and neutrons are made up of one upper quark and two lower quarks.
Different quark drops are combined by "gluon" transporting strong forces (strong interactions), and in fact, the masses of the three quarks account for less than 1% of the proton (neutron) mass, and the other 99% of the masses are the masses produced by the strong interaction.
Gluons, like photons that propagate electromagnetic forces, are propagators and have no mass (static mass) themselves.
So can smaller quarks continue to be subdivided?
Scientists don't know yet. Because quarks are confined, quarks can't exist on their own, and scientists can't even observe quarks, at least not at the moment, let alone subdivide them.
A more advanced theory, string theory, holds that all elementary particles such as quarks are made up of vibrating strings, and that different vibrational directions and frequencies make up different elementary particles. In addition to string theory, there are theories of membrane universes, high-dimensional space-time, multiverse, and so on.
However, the above theories are difficult to verify, and they are currently more stuck in the stage of reasoning and conjecture.