In recent years, there have been a number of breakthroughs in the field of tumor treatment, and a number of star "anti-cancer families" have been born, such as the well-known PD-1/PD-L1 family (multiple cancers), EGFR family (lung cancer), ALK family (lung cancer), etc., and countless cancer patients have obtained "new life".
In addition, there is a rising star of anti-cancer - the PARP family, especially in the last six months, a number of blockbuster progress has been announced:
In September 2019, during the European Annual Conference of Clinical Oncology (ESMO), four major PARP inhibitors published exciting clinical data, all of which showed excellent efficacy in patients with ovarian cancer without considering BRCA mutational states;
In October 2019, Nilapally received a new approval from the U.S. FDA for the treatment of patients with ovarian cancer with at least 3-line failure of advanced homologous recombination repair defects;
In December 2019, nilapally was approved domestically for maintenance therapy for recurrent ovarian cancer, the second approved PARP inhibitor in China after olaparelli;
In December 2019, Orapali received a new approval from the U.S. FDA for single-agent, first-line maintenance treatment of patients with metastatic pancreatic cancer with inherited BRCA blastocryonic mutations and no disease progression for at least 16 weeks with a platinum-containing regimen.
Recently, a number of domestic PARP inhibitors have obtained clinical trial approval from the State Food and Drug Administration, and clinical trials have been carried out in China for many solid tumors such as ovarian cancer, breast cancer, prostate cancer, pancreatic cancer and so on.
In view of this, Tongtong summarizes the latest progress of PARP inhibitors at home and abroad for the majority of cancer patients, and also expects these new drugs to bring hope to more patients.
<h1 class="pgc-h-arrow-right" > marketable PARP inhibitors</h1>
(1) Olaparib: domestically listed, into medical insurance
Olapally is the world's first marketed PARP inhibitor developed by AstraZeneca, and since it was approved for marketing in the United States at the end of 2014, it has now covered the first and second-line treatment of BRCA patients with mutant ovarian cancer and the second- and third-line treatment of refractory recurrent breast cancer and the first-line treatment of BRCA mutant pancreatic cancer.
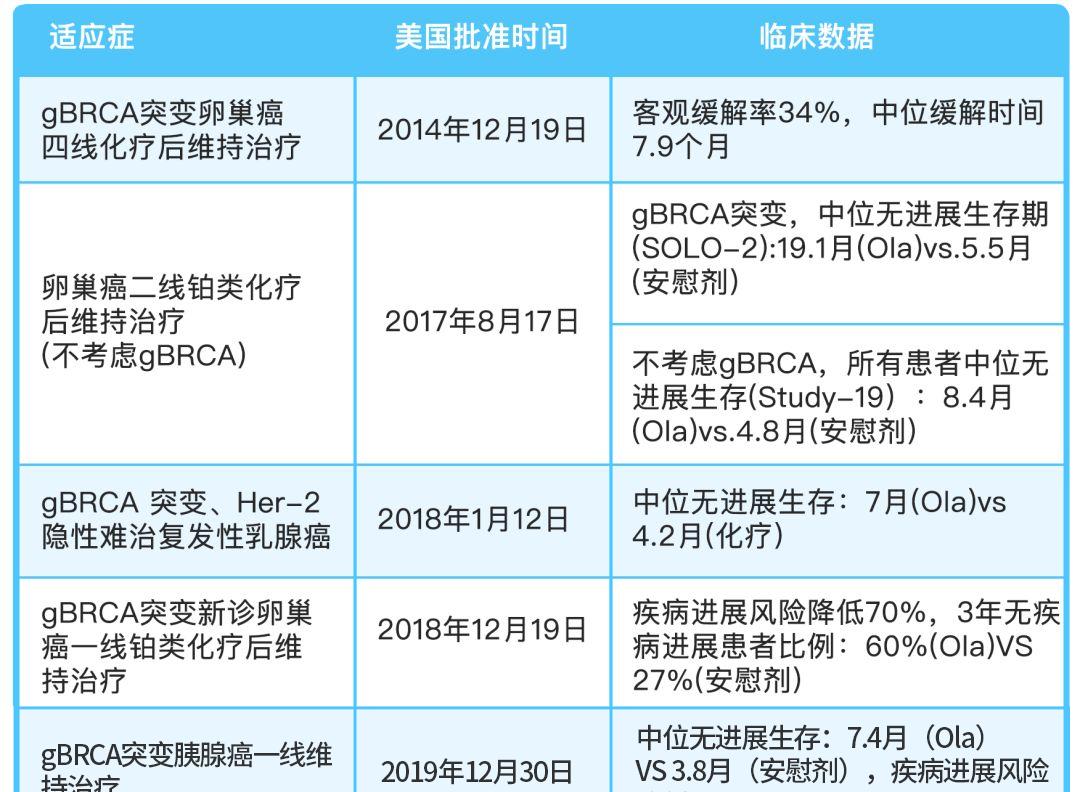
At present, Olapalli has been listed in China on August 22, 2018, and the approved indications include: maintenance therapy after second-line platinum chemotherapy for recurrent ovarian cancer regardless of gBRCA mutation status; first-line maintenance therapy for brCA mutant advanced ovarian cancer.
For details, please refer to:
Ten years of grinding a sword, Orapali officially landed in China, and ovarian cancer treatment ushered in a major breakthrough
Precision sniping, and see how Olapali kills cancer cells neatly, 6 top international magazines
What is more worth mentioning is that Olapalli has been successfully included in the scope of Class B drugs in the 2019 National Medical Insurance Catalogue. Orapali has also brought an important breakthrough to targeted therapy for ovarian cancer patients in China. For details, the targeted drug olapal for ovarian cancer entered the national medical insurance directory
(2) Rikapally Rucaparib
Ricapalli is the second marketable PARP inhibitor developed by Clovis, but it is the first PARP inhibitor in the world to be accelerated to be approved for the third-line treatment of ovarian cancer.
(3) Niraparib: Listed in China
Nilapally is the third parp inhibitor jointly developed by Tesaro and GSK, and although it was listed in the United States 3 years after Oolapally, it has progressed rapidly as a dark horse among PARP inhibitors, and has also created the first in two therapeutic areas:
The first approval is for maintenance therapy after second-line platinum chemotherapy for recurrent ovarian cancer, regardless of whether there is a gBRCA mutation.
The first approval is for fourth-line and above treatment in patients with HOMOlogous recombinant defect HRD-positive ovarian cancer.
Currently, indications and clinical data of Nilapally abroad include:
Zaiding Pharmaceutical has introduced nilapally into China, is conducting clinical trials in the two fields of small cell lung cancer and ovarian cancer, submitted a listing application to the China Drug Review Center in December 2018, and on December 27, 2019, Nilapali was approved by the Food and Drug Administration and officially landed in China. At present, patient assistance projects have also been introduced, and the price is close to the people.
(4) Thaprazole Pali Talasoparib
Tapazopari is the fourth globally marketed PARP inhibitor developed by Pfizer, and the first approved indication is breast cancer with gBRCA mutation, and the specific clinical data are as follows:
<h1 class="pgc-h-arrow-right" > unlisted PARP inhibitor</h1>
In addition to the PARP inhibitors that have been listed on the market, there are new faces that are not listed that are worth looking forward to.
(1) Velipali Veliparib
Abbott's Velipali may be the fifth PARP inhibitor on the market in the world to present the results at the 2019 ESMO conference to publish the results of maintenance treatments for ovarian and breast cancer.
(2) Fluzopali Frizoparib
Fluzopali is a PARP inhibitor developed by Hengrui Pharmaceutical, and has been included in the priority review of listed drugs by the China Drug Review Center, and it is speculated that the indications for marketing are most likely to be patients with recurrent ovarian cancer, which has been promoted to the phase III clinical stage in China. In other words, this may be the first Chinese indigenous PARP inhibitor to be listed in China.
Fluzopali also presented excellent trial results at the ESMO conference in 2019: in a multicenter Phase IB study exploring efficacy and safety in PLATINUM-sensitive ovarian cancer with BRCA1/2 mutations, the ORR was as high as 55.8%, and as of the date of publication of the data, the median PFS and OS had not been reached, which implied a sustained survival advantage.
③ Pamiparib
Pamiparib's research and development progress has also attracted much attention, because it is the company that has set a record for the first successful listing of a new drug in China in the United States, and the PARP inhibitor developed by BeiGene is currently conducting key trials of ovarian, prostate and stomach cancers in countries including China.
The results of a Phase I clinical trial of Pamiparib monotherapy and low-dose temozolomide combined with low-dose temozolomide for safety and dose exploration in patients with solid tumors were presented at the ESMO conference in 2019, showing that they were safe and achieved some degree of disease remission in patients with multiple solid tumors.
In just 5 years, PARP inhibitors have become one of the important treatment methods for ovarian cancer and breast cancer, and at the same time, they have shown excellent clinical data in cancers including cancer king pancreatic cancer, which is more hopeful for patients.
Looking forward to more drugs, more affordable prices!
Resources
[1] Domchek, Susan M., et al. “Efficacy and Safety ofOlaparib Monotherapy in Germline BRCA1/2 Mutation Carriers with AdvancedOvarian Cancer and Three or More Lines of Prior Therapy.” Gynecologic Oncology,vol. 140, no. 2, 2016, pp. 199–203.
[2] Pujade-Lauraine, Eric, et al. “Olaparib Tabletsas Maintenance Therapy in Patients with Platinum-Sensitive, Relapsed OvarianCancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Double-Blind, Randomised,Placebo-Controlled, Phase 3 Trial.” Lancet Oncology, vol. 18, no.9, 2017, pp. 1274–1284.
[3] Moore, Kathleen, et al. “Maintenance Olaparibin Patients With Newly Diagnosed Advanced Ovarian Cancer.” Obstetrical& Gynecological Survey, vol. 74, no. 2, 2019, pp. 86–87.
[4] LBA3 – Coleman RL, Fleming GF, Brady MF, etal. VELIA/GOG-3005: Integration of veliparib (V) with front-line chemotherapyand maintenance in women with high-grade serous carcinoma of ovarian, fallopiantube, or primary peritoneal origin (HGSC).
[5] Ledermann J, Harter P, Gourley M, et al. Olaparib maintenance therapy inplatinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-1392.
[6] Moore, Kathleen, et al. “Maintenance Olaparibin Patients With Newly Diagnosed Advanced Ovarian Cancer.” Obstetrical& Gynecological Survey, vol. 74, no. 2, 2019, pp. 86–87.
[7] Moore, Kathleen N., et al. “NiraparibMonotherapy for Late-Line Treatment of Ovarian Cancer (QUADRA): A Multicentre,Open-Label, Single-Arm, Phase 2 Trial.” Lancet Oncology, vol. 20,no. 5, 2019, pp. 636–648.
[8] Coleman, Robert L., et al. “Rucaparib MaintenanceTreatment for Recurrent Ovarian Carcinoma after Response to Platinum Therapy(ARIEL3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial.” TheLancet, vol. 390, no. 10106, 2017, pp. 1949–1961.
[9] Litton, Jennifer K., et al. “Talazoparib inPatients with Advanced Breast Cancer and a Germline BRCA Mutation.” TheNew England Journal of Medicine, vol. 379, no. 8, 2018, pp. 753–763.
[10] LBA9 – Diéras VC, Han HS, Kaufman B, etal. Phase 3 study of veliparib with carboplatin and paclitaxel inHER2-negative advanced/metastatic gBRCA-associated breast cancer.
[11] LBA3 – Coleman RL, Fleming GF, Brady MF, etal. VELIA/GOG-3005: Integration of veliparib (V) with front-linechemotherapy and maintenance in women with high-grade serous carcinoma ofovarian, fallopian tube, or primary peritoneal origin (HGSC).
[12] Voskoboynik, M., et al. “452PDSafety, AntitumorActivity, and Pharmacokinetics (PK) of Pamiparib (BGB-290), a PARP1/2Inhibitor, in Patients (Pts) with Advanced Solid Tumours: Updated Phase IDose-Escalation/Expansion Results.” Annals of Oncology, vol. 30,2019.
[13] Stradella, A., et al. “451PDUpdated Results of the PARP1/2 InhibitorPamiparib in Combination with Low-Dose (Ld) Temozolomide (TMZ) in Patients(Pts) with Locally Advanced or Metastatic Solid Tumours.” Annals ofOncology, vol. 30, 2019.
[14] N li, l Wu, Y Zhang, J Liu, Q Zhou, J Zhu, Ryin, L Wang, G Li, X Wu, H Pan, S Yao, Q Wu, K Gu, H Zhang, X Wan, R An, J Zou,Q Wang, 1000P
Efficacy and safety of oral poly (ADP-ribose) polymerase inhibitor fluzoparibin patients with BRCA1/2 mutations and platinum sensitive recurrent ovariancancer, Annals of Oncology, Volume 30, Issue Supplement_5, October 2019