▎ WuXi AppTec content team editor
Recently, Applied Molecular Transport (AMT) announced that its phase 2 clinical trial of oral IL-10 cytokine research therapy AMT-101, a single drug for patients with chronic marsupialitis ( a refractory inflammatory bowel disease ) , has achieved positive top-line results. The Independent Data Monitoring Board (DMC), based on a review of safety and efficacy data, recommends that AMT-101 be advanced to a Phase 3 clinical study to treat chronic chagalitis at a dose of 3 mg.
Nearly 30% of patients with ulcerative colitis (UC) eventually require complete removal of the colon. Undergoing an anal anastomosis called ileal anastomosis (IPAA) allows patients to be able to remove waste normally after removal of the colon and rectum. Then inflammation may occur in the walls of the pouch that remains after surgery, called pouchitis. The clinical manifestations of marsupialitis are excessive defecation, urgency, fecal incontinence, nocturnal exudate, and lower abdominal pain. There are currently no approved treatments in the United States. Acute marsupialitis usually responds to antibiotic therapy, but up to 50% of patients with marsupialitis develop chronic marsupialitis, in which patients often relapse or do not respond to antibiotic therapy.
AMT-101 is a novel gastrointestinal selective, oral IL-10 therapy. AMT engineered the Cholix protein secreted by Vibrio cholerae to become a vehicle to assist biologics in crossing intestinal epithelial disorders. By binding to receptors on the surface of the intestinal epithelium, the Cholix protein is able to use endocytosis to pass through epithelial cells into the bloodstream. AMT-101 combines a vector developed based on the Cholix protein with IL-10 to form a fusion protein that assists IL-10 to cross the intestinal epithelium and aggregates at the site of intestinal inflammation, potentially avoiding possible side effects of systemic administration. It is currently being used in four Phase 2 clinical trials for the treatment of chronic marsupialitis, ulcerative colitis and rheumatoid arthritis (RA).
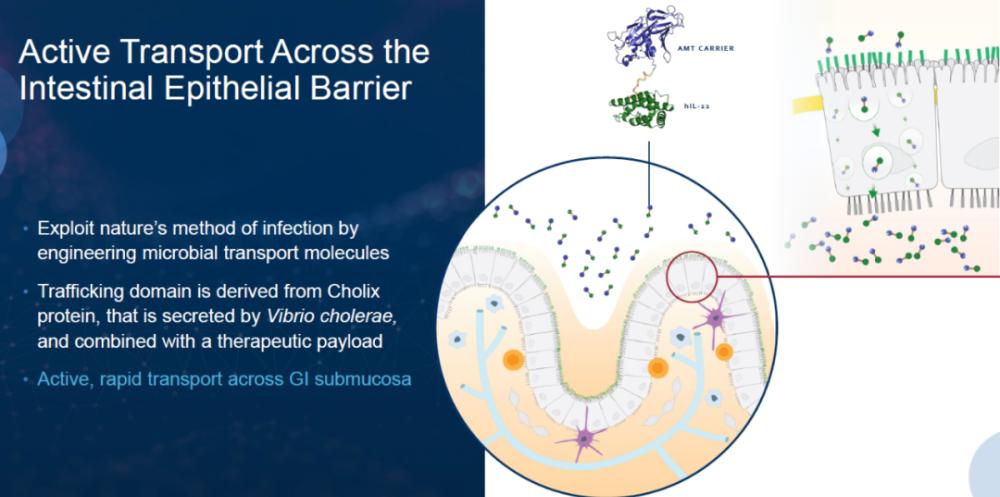
▲AMT company's carrier technology (image source: AMT company's official website)
This double-blind Phase 2 clinical trial included 22 patients with chronic pouchitis randomly assigned to receive treatment with 3 mg or 10 mg of oral AMT-101. The test results showed:
Combining patient data from both dose groups, the frequency of bowel movements improved in 36.4% (8/22) of patients. As early as week 2, frequent responses to bowel movements were observed in both dose groups and persisted throughout the duration of treatment. Mid-threshold data showed improvement in other symptoms such as urgency to defecate, urinary incontinence, and abdominal cramps. The proportion of patients in both dose groups who achieved improvement in symptomatic bowel frequency exceeded the criteria for advancing the project to a Phase 3 clinical trial.
In terms of histological testing, 22.7% (5/22) of patients achieved a pre-set Geboes score ≤ 3.1 histological healing response, an objective assessment of disease improvement. The patient's median baseline Geboes score was 5.1, indicating severe pouchitis with ulceration and tissue destruction. Both dose groups showed histological healing responses.
▲AMT-101 key efficacy endpoint results (Image source: Reference[1])
AMT-101 observed good safety and tolerance. Most of the adverse events were mild to moderate, with only 1 serious adverse event observed as cytomegalovirus (CMV) infection, which was determined to be unrelated to the drug under study.
"We are excited to share this data with the U.S. FDA and other regulatory agencies to advance the development of AMT-101 in chronic marsuponitis." Bittoo Kanwar, MD, Chief Medical Officer of AMT, said, "We believe these data support the potential of AMT-101 in the treatment of mucosal immunology and inflammation-related diseases. ”
Resources:
[1] Applied Molecular Transport Announces Positive Top-line Phase 2 Results from FILLMORE Trial of Oral AMT-101 in Patients with Chronic Pouchitis. Retrieved April 25, 2022, from https://ir.appliedmt.com/news-releases/news-release-details/applied-molecular-transport-announces-positive-top-line-phase-2
Disclaimer: WuXi AppTec's content team focuses on the global biomedical health research process. This article is for informational purposes only and the views expressed herein do not represent the position of WuXi AppTec, nor do they represent WuXi AppTec's support for or opposition to the views expressed herein. This article is also not recommended for treatment options. For guidance on treatment options, please visit a regular hospital.
Copyright note: This article is from WuXi AppTec content team, welcome to forward to the circle of friends, refuse the media or institutions to reprint to other platforms in any form without authorization. Reprint authorization, please reply to "Reprint" on the "WuXi AppTec" WeChat public account to obtain the reprint instructions.
Share, like, watch, focus on global biomedical health innovation