Introduction: On April 8, 2022, Zhijian Jinrui first announced its self-developed second-generation RET inhibitor APS03118 preclinical key research results at the annual meeting of the American Association for Cancer Research (AACR), which showed that APS03118 is a highly selective second-generation RET inhibitor, and in vivo and in vitro tests have shown a strong inhibitory effect on a variety of RET gene variants, which can be used in patients who are resistant to first-generation selective RET inhibitors.
Image source: AACR official website
APS03118 is an innovative drug independently developed by Zhijian Jinrui with innovative structure and global independent intellectual property rights, mainly for patients with non-small cell lung cancer, thyroid cancer, breast cancer, colorectal cancer and other advanced solid tumors caused by RET gene changes.
The pathogenesis of RET gene is mainly gene mutation and gene fusion, which can lead to excessive activation of the RET signaling pathway, thereby participating in the proliferation, apoptosis and invasion of different tumor cells, which in turn affects the occurrence and progression of tumors. Since the RET oncogene is present in many cancers, it has become one of the important signaling pathways for "unlimited cancer" therapies. Tumors carrying mutations in the RET gene rely primarily on abnormal activation of this protein kinase to promote their proliferation and growth, so these tumors are very sensitive to RET inhibitors.
Although the RET gene is closely related to the occurrence of multiple tumors in humans, only selpercatinib and prasetinib have been approved for marketing with the RET gene as a target, which has a good therapeutic effect on RET-positive patients, but then it is inevitable that drug resistance and disease progression will occur. The key findings presented by APS03118 at the AACR conference [1] show that the main resistance mechanisms of the first-generation selective RET inhibitors include the roof mutation RET L730I/M, the goalkeeper mutation RET V804M/L/E and the hinge mutation RET Y806H in addition to the solvent frontier mutation (SFMs). The second-generation RET inhibitor APS03118 is able to have a stronger inhibitory effect on solvent frontier mutations and a range of other mutations that lead to resistance to first-generation RET inhibitors. The key figures are as follows:
APS03118 is a highly selective second-generation RET inhibitor
In the Eurofins ScanMax test, APS03118 tested 468 kinases at a concentration of 100 nanomol compounds, which is highly selective for RET. In the Eurofins Safety47 test, there was little off-target safety risk at 10 micromolal compound concentrations.
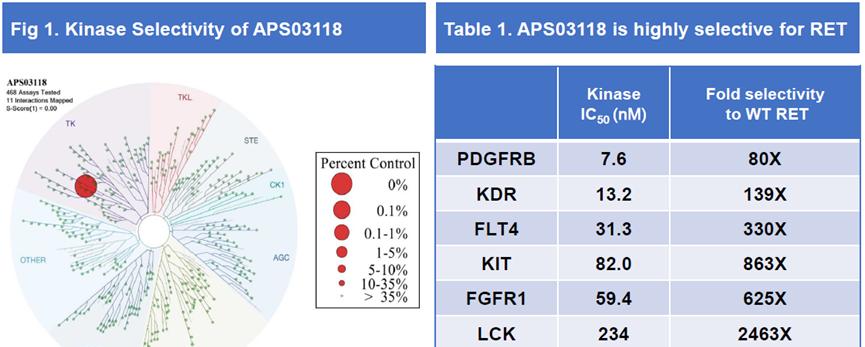
APS03118 has a strong inhibitory activity against RET gene alterations in in vitro assays
In enzyme and cell assays, APS03118 was effective against wild-type (WT) and a range of RET mutations and fusions compared to selpercatinib and prasetinib, particularly against solvent frontier mutations (SFMs) RET G810R/S/C, rooftop mutations RET L730I/M, gatekeeper mutations RET V804M/L/E and hinge mutations RET Y806H, compared to selpercatinib and prasetinib.
APS03118 has potent tumor suppressor activity in mouse xenograft tumor models
APS03118 has potent tumor suppressor activity in mouse xenograft tumor models such as KIF5B-RETPDX, KIF5B-RET V804M CDX, and KIF5B-RETG810R CDX. In the CCDC6-RET in situ graft PDX model, the tumors in the APS03118 treatment group completely regressed, and the mouse survival rate was 100%.
RET gene is closely related to the occurrence of a variety of human tumors, of which lung cancer is the malignant tumor with the highest incidence and mortality in mainland China, non-small cell lung cancer is the most common type of lung cancer, non-small cell carcinoma patients with RET gene changes in clinical practice are not uncommon, in addition, lung cancer patients have a high incidence of brain metastasis, of which RET fusion positive is closely related to the high risk of brain metastasis, and the cumulative incidence of 24 months is greater than 60% [2]. APS03118 can provide a better treatment plan for non-small cell lung cancer, thyroid cancer and other cancers caused by RET gene modification.
Dr. Zhong Jun, Vice President of R&D of Zhijian Jinrui, said: "In preclinical research, APS03118 has proved its great potential in the treatment of cancer with RET gene alteration, and this AACR conference hopes to make more people pay attention to RET target therapy, for the global unmet clinical needs in this field, to provide better treatment options for cancer patients, and to provide more treatment options for patients who are resistant to the first generation of selective RET inhibitors." ”
Earlier this year, the APS03118 Clinical Trial Application (IND) was approved by the U.S. Food and Drug Administration (FDA)[3] and was granted a fast-track qualification by the U.S. FDA[4]. APS03118 presented key preclinical findings at the AACR conference, adding more confidence to the global clinical trials program being launched.
Beijing Zhijian Jinrui Biomedical Technology Co., Ltd. is a biomedical high-tech company focusing on the precision treatment of cancer, focusing on the research and development of tumor-driven gene drugs through in-depth exploration of oncogenes, protein structure and function, and synthetic medicinal chemistry. Zhijian Jinrui aims to explore life sciences, innovate precision cancer treatment, and seek new hope for cancer patients around the world with breakthrough precision oncology therapies.
Typography | essay competition
End
Resources:
[1] Alexander Drilon, Jun Zhong, Ying Lu, et al. Proceedings of the 113th Annual Meeting of the American Association for Cancer Research; 2021 April 8-13; New Orleans LA. Philadelphia(PA): AACR; 2022. Abstract nr .
[2] Jiyun Lee, Bo Mi Ku, Joon Ho Shim, et al, Characteristics and outcomes of RET-rearranged Korean non-small cell lung cancer patients in real-world practice, Japanese Journal of Clinical Oncology,2020,50(5)594–601.