People's Daily health client Sun Huan
On March 1, the official website of the State Food and Drug Administration released the news that the World Health Organization said that the Sabin strain (Vero cell) inactivated polio vaccine (sIPV) of sinopharm's Beijing Institute of Biological Products in China was pre-certified and can be procured by the UNITED Nations system.
People's Daily health client noted that since May 2013, Sinopharm Zhongsheng Co., Ltd. Chengdu Company Japanese encephalitis live attenuated vaccine through the WHO pre-certification site inspection, to achieve the "breakthrough of zero" in the internationalization of Chinese vaccines, in recent years, there have been vaccines to the international, the Sinopharm polio inactivated vaccine is the seventh Chinese domestic vaccine to pass the pre-certification.
Currently, it has passed the WHO pre-certified Chinese vaccine
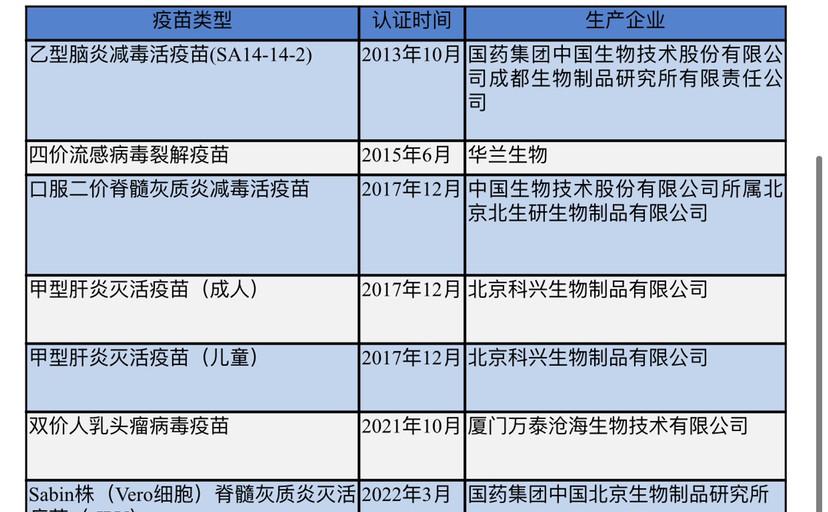
Relevant data show that polio is a highly contagious disease caused by a virus, mainly affecting young children. The virus spreads through contaminated food and water, multiplies in the intestines, and then invades the nervous system. In rare cases, the disease can cause permanent paralysis.
WHO Pre-Certification is a United Nations action plan launched in 2001 to expand the choice of priority medicines, with the goal of ensuring the quality, efficacy and safety of medicines procured by international funds for patients in developing countries.
The State Food and Drug Administration said that China's biological polio inactivated vaccine has passed who pre-certification, marking that the supervision, development and production system and product quality of China's vaccine products have been internationally recognized, and will also make a significant contribution to the global eradication of polio diseases.