Organ transplantation is the process of transferring an organ from one individual to another, although it sounds simple, but in our cognition, the conditions of organ transplantation are very harsh. The human immune system has a very perfect defense mechanism against various pathogenic factors, and it will also recognize foreign transplanted organs as "alien components" and undergo an immune response, which is transplant rejection. Transplant rejection is a complex immunological phenomenon, mainly due to the difference in receptor and graft human leukocyte antigen HLA (human leukocyte antigen), so blood and HLA matching is required before organ transplant surgery.
The University of Maryland Medical Center announced on January 10, 2022, that it had successfully transplanted a "genetically modified pig heart" into a 57-year-old male patient with severe heart failure and arrhythmias.
Replacing "pig heart" for "human heart" sounds a bit out of line...
This pig heart is not simple
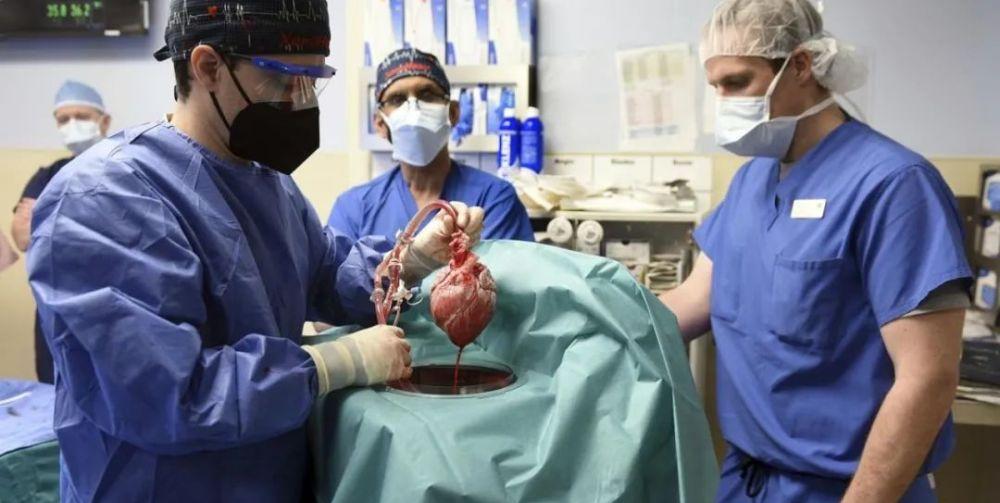
The recipient of this organ transplant, David Bennett, could not accept the mechanical heart pump due to his irregular heart rhythm, and Bennett had a "previous crime" of not complying with the doctor's advice, nor could he accept human transplantation, and could only stay in bed and rely on the life support system to survive. On December 31, 2021, the US Food and Drug Administration (FDA) urgently approved Bennett for a swine heart transplant, giving him a chance to be "reborn".
The surgical team that performed the swine heart transplant was also prepared, and before that, the team of surgeon Muhammad Mohiuddin of the University of Maryland Medical Center had also conducted several trials of pig heart transplantation in baboons. The trial went well, the baboons had normal vital signs after surgery, and the pig-derived heart was able to function normally.
Of course, the core of the operation - "pig heart" is not simple. Because of the existence of the immune system, the human body will definitely reject the pig heart, and only so that the immune system cannot recognize the foreign pig heart can it be confused. The pig heart was genetically edited to knock out the sugar chain code (GGTG1), two sugar chain modification genes (CMAH and B4GALNT2) on the pig cells and the Class I pig leukocyte antigen (SLA) that presented antigen information, while editing multiple human immunosuppressive genes to reduce immune rejection, more than 10 gene editing techniques to make the heart as unrecognizable as possible by the immune system.
At the same time, in order to avoid the pig-derived heart from bringing in some virus from pigs, this pig heart comes from PERV-free (porcine endogenous retrovirus) pigs. PERV is a viral gene in the pig-based group, and basically all pigs have PERV.
David Bennett's transplant went well and "heart function looks great". Muhammad Mohiuddin and his team will continue to monitor David Bennett's immune response and heart function.
Admittedly, the success of the surgery also requires continued attention to David Bennett's prognosis and health, and it is too early to determine the success of the swine heart transplant.
Back to the pigs themselves
Human research on xenotransplantation began in 1902, when people tried to transplant pigs, sheep, monkeys, etc. as organ donors, but they were limited to the medical and scientific level at the time, and all ended in failure; in 1963, baboon-derived kidney transplant patients survived 19 to 98 days after surgery; in 1964, the first animal-derived heart transplant patients survived for 2 hours and died; in 1983, a newborn survived 20 days after receiving a baboon-derived heart transplant...
In August 2017, Hubei Xiangya Third Hospital also had a case of "porcine cornea transplantation". Although in this case, the cornea of the pig is only a temporary substitute for the suitable cornea, in the field of xenotransplantation, the appearance rate of pigs gradually increases.
In fact, the physiological structure, metabolism and human proximity of non-human primates such as monkeys, orangutans, baboons and other animals, why do cross-species organ transplants choose pigs as donors?
First, non-human primates are closely related to humans, and many diseases can be transmitted to each other. Moreover, primates have low reproductive rates, long intergenerational gaps, and high feeding costs, making it difficult to become a stable source of organs.
Secondly, the size of the pig's organs is just right, and the anatomy, physiological indicators, blood type antigens, etc. are similar to humans. At the same time, pigs have a short growth cycle and are easy to raise. The "Galsafe pig" bred by the American regenerative medicine company Revivcor is genetically modified to contain sugar molecules that do not contain α-gal, which can prevent acute rejection after transplantation into the human body. In September 2021, the company's Galsafe pigs were also used as kidney donors for human surgery, and the pig-derived kidneys had no obvious rejection reaction in the patient's body.
There are research institutes around the world that study gene-edited pigs, all of which are designed to promote cross-species transplantation of pig-derived organs to alleviate the current dilemma of organ shortages worldwide. Hunan Xenotransplantation Engineering Research Center in Mainland China is the world's second medical-grade donor pig breeding center.
The Chinese female scientist who "designed" the pig
The pig heart that continues to beat in David Bennett's body is the product of gene editing technology, which is backed by the contribution of a Chinese female scientist.
Known as the "gene scissor hand", Yang Luhan's research project is how to modify 62 genes, that is, endogenous retrovirus PERV, on a single cell of pigs, while ensuring the integrity of the genome. In 2017, Yang Luhan led the team to successfully use CRISPR-Cas9 gene editing technology to overcome this problem, which greatly improved the efficiency of gene editing and shortened the time to breed transgenic pigs.
It is the contribution of Yang Luhan's team CRISPR-Cas9, a gene knockout technology, that fundamentally solves the risk that pig organ transplantation into the human body may lead to viral infection.
In December 2021, Yang Luhan also participated in a preclinical study of polygenic edited porcine-monkey heart, liver and kidney transplantation published in Organ Transplantation. The study explores the application prospects of gene-edited pigs in xenotransplantation. The results of the study show that transgenic pigs have certain advantages in overcoming ultra-acute rejection, alleviating humoral rejection and coagulation disorders, but whether they can be used as potential donors for clinical xenotransplantation needs to be further evaluated.
Resources:
Nature:First pig-to-human heart transplant: what can scientists learn?
Xin Zhiyuan: There is a Sichuan girl behind the pig heart transplantation human body! 36-year-old Harvard female school bully knocked out the disease-causing gene of pigs and was praised as "gene scissor hand"
Public network: 27-year-old man transplanted pig cornea vision has been restored, or there is rejection
Edit: Ge Ge Wu