Source: 21st Century Business Herald, Nanfang +, CCTV News, The Paper, Jiangxi Children's Hospital, Chengdu Daily Jinguan News, etc
On the first day of 2022, the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalogue (2021) was officially implemented.
According to the new version of the national medical insurance drug catalogue, a total of 74 drugs have been added to the catalogue, and 11 drugs have been transferred out of the catalogue. In 2021, the total number of drugs in the national medical insurance drug list is 2860.
In addition, according to the 21st Century Business Herald, on the first day of the landing of the new version of the national medical insurance drug catalog, nearly 20 patients with spinal muscular dystrophy (SMA) in hospitals in 11 provinces and cities such as Beijing, Shanghai, Guangdong, Zhejiang, Shandong, Hunan, Hubei, Fujian, Jiangxi, Henan and other provinces and cities received the most concerned Bojian "700,000 one injection" treatment of spinal cord muscular atrophy (SMA) drug sodium injection and treatment, the drug price was reduced to 10,000 yuan, greatly reducing the economic burden of the corresponding groups.
On the first day of the New Year, many places began to fight
Patient: "I feel lucky to explode"
On the first day of the New Year in 2022, the Department of Neurology of Jiangxi Children's Hospital conducted nocinnassine sodium treatment for 2-year-old and 10-month-old children with SMA, Jiacheng (pseudonym), sending the best New Year gift to SMA patients, which is the first case in our province after the SMA targeted drug was included in medical insurance, and it is also the first batch of patients in the country to benefit.
According to Nanfang +, at about 9 a.m. on January 1, in the treatment room of the Department of Neurology of the First Affiliated Hospital of Sun Yat-sen University in Guangzhou, Mr. Du, a 29-year-old patient, picked up his clothes and lay on his side on the bed, preparing to undergo a lumbar puncture. What was about to be injected into his body was Nosinasin sodium.
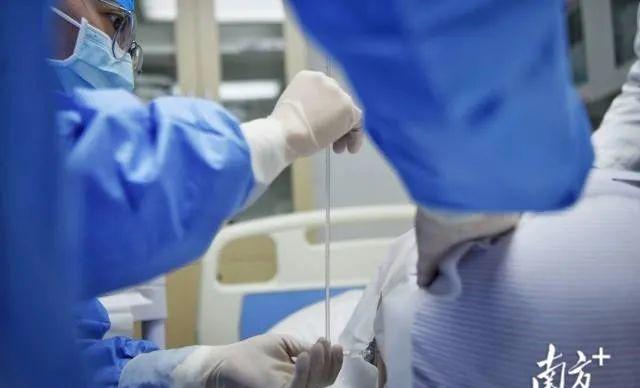
lumbar puncture
"Lean your head a little closer to your chest and arch your back a little more." Li Jing, attending physician of the Department of Neurology of the First Affiliated Hospital of Sun Yat-sen University, gradually guided Mr. Du to adjust his position. Mr. Du lay on the left side of the bed, bent knees and hugged the group, exposing the lumbar spine.
"The back and the bed should be kept as vertical as possible, and when the needle is pierced, it will be slightly oblique and slowly upward..." Zhang Cheng, chief physician and professor of the Department of Neurosurgery of Zhongshan First Hospital, came to the scene for guidance.
On the other side, doctor Xu Ruikai is already ready to go in the hospital pharmacy. He had an incubator in his hand containing a sodium injection of Nosina raw sodium that was cryopreserved at low temperatures. "You can bring the medicine." It didn't take long for him to receive instructions to deliver the medicine.
The tip of the needle entered the body, and Mr. Du tightened his fist. "Relax a little, breathe normally, and don't strain your stomach." The paramedics continued to patiently guide.
After the lumbar puncture was completed, the attending physician He Ruojie took the injection out of the cold incubator, held it in the palm of his hand, and warmed the injection to about 25 degrees Celsius. After 2 minutes, the temperature gun showed that the temperature of the injection was suitable.
Li Jing and He Ruojie cooperated and used a syringe to suck out 5 ml of Nocina raw sodium injection from a small bottle. "This step should be taken with special care, every drop of medicine is precious, and try to suck it into the needle tube."
After all the suction was taken out, Li Jing slowly injected the injection into Mr. Du's body and completed the treatment.
"I can use this medicine on the first day of the new year, and I feel lucky enough to explode!" Mr. Du is full of hope for the future, "I hope that I can recover my health in the future, go to work normally, live a normal life, and hope that more patients, especially children, can recover their health." ”
Doctors give injections to patients
Northinacana injection
At the same time, the reporter came to the Binjiang Branch of the Children's Hospital Affiliated to Zhejiang University School of Medicine (hereinafter referred to as "Zhejiang University Children's Hospital") on the morning of the 1st, and on the 18th floor of the neurology department of the hospital's inpatient department, Xinxin, a 4-year-old child with SMA, was waiting for the injection of Nocinasine sodium.
At 10:36 a.m., Xinxin was carried into the injection room; at 10:48 a.m., the doctor's team pushed open the door and the whole process ended
Mao Shanshan, chief physician of the Department of Neurology of the Children's Hospital Affiliated to Zhejiang University School of Medicine, leader of the multiscientific diagnosis and treatment team for spinal muscular atrophy (SMA-MDT), and also the leader of the SMA diagnosis and treatment expert group in Zhejiang Province, introduced to reporters that Xinxin was diagnosed with SMA TYPE II at the age of 1 year and 9 months, and had been receiving management and rehabilitation treatment from the multi-scientific diagnosis and treatment team. ”
"Every small group should not be abandoned"
"I hope that the company will come up with a more sincere offer... Every small group should not be abandoned. ”
In the past, the high price of Nosinasin sodium injection made it difficult for many patients, including Mr. Du and Xinxin, to reach.
But now it's different. At the end of 2021, a video of a health care negotiator negotiating a price negotiation with a company representative on a rare disease drug came into attention. In the video, the medical insurance negotiators "killed" the price of the drug from the market price of 700,000 yuan to about 30,000 yuan, which triggered netizens to like it.
In this year's negotiations, negotiations for drugs to treat the rare disease spinal muscular atrophy were conducted for an hour and a half,
The whole process of review can be described as extremely difficult, the first round of quotations of the company, the price given is 53680 per bottle. Zhang Jinni, a negotiator of the National Medical Insurance Bureau, replied: "I hope that enterprises will come up with more sincere offers, and every small group should not be abandoned." ”
And gave a rather "tempting" condition - "if this drug can enter the medical insurance list, with China's population base, the Determination of the Chinese Government to serve patients, it is difficult to find such a market." ”
After the representative of the enterprise left the table for consultation. The offer given dropped to 48,000 yuan per bottle.
Zhang Jinni said frankly that China's medical insurance fund this year is actually a very difficult year, including the early stage of the new crown pneumonia, the reduction and reduction of the medical insurance fund may also enjoy the corresponding preferential treatment, vaccine costs actually account for a very, very large expenditure of the medical insurance fund.
"Therefore, we still have the courage to carry out medical insurance negotiations this year, and we really feel the great determination of the people's health first."
Zhang Jinni's words made the company representative leave the table again for discussion.
This time they lowered the price to 45,800 yuan.
The reply given by Zhang Jinni was: "It is very difficult, and I hope that the company will work hard again." ”
After the negotiation company left the table for the third time, it brought back the price of 42800.
This time, Zhang Jinni replied: "I believe that the company feels very painful, but there is still a certain distance from us to talk further." ”
The negotiating company experienced the fourth and fifth departure negotiations, and the price dropped to 37800 per bottle. But this is clearly not up to the ideal price point for the Medicare.
Zhang Jinni smiled and said: "At the negotiating table, as Party A, it is really difficult to be so humble."
But the negotiating team's bottom line on price is not compromising.
"Adjust space is 0" "It's really tough, I think I just burst into tears. ”
Subsequently, after the collective deliberation of the negotiation team, Zhang Jinni gave an offer of 33,000.
The representative of the enterprise left the table for the eighth time and finally confirmed the new offer:
"Is this the final offer you confirmed?"
"Confirm."
"Okay, deal."
After a long negotiation of one and a half hours, the representatives of the enterprise left the table eight times to negotiate. The transaction price of each bottle of medicine is more than 20,000 yuan less than the initial offer.
The drug that was knocked down at this price is Nocinasin sodium injection, which is also the first time that a rare disease drug of value has been included in the medical insurance drug list, and it will be officially implemented from January 1, 2022.
This means that more children with SMA can afford to use medicines, which also greatly reduces the financial burden on the patient's family, and the patient's family members are overjoyed and cry after learning the news!
Xing Huanping, executive director of Mei'er SMA Care Center, said: "We have witnessed the patient group from no drug available, to the hope of treatment, to today's medical insurance policy, and the family burden has been greatly reduced. This is the best New Year's gift that SMA patients and families across the country have ever received. ”
Why is Nosinasin sodium so expensive?
SMA, or spinal muscular atrophy, is an autosomal recessive disorder caused by genetic defects and one of the most common genetic disorders that cause infant death.
Professor Huang Shaoping, chief expert of the Children's SMA Diagnosis and Treatment Center of the Second Affiliated Hospital of Xi'an Jiaotong University and head of the discipline of the pediatric treatment team, said that SMA is a rare disease caused by degeneration of motor neurons in the anterior horn of the spinal cord, resulting in muscle weakness and muscle atrophy, with an incidence of about one in 10,000.
It is understood that in May 2018, spinal muscular atrophy was included in the "First Rare Disease Directory" jointly formulated by the National Health Commission and other departments, which is the second largest neuromuscular rare disease.
Jin Ruifeng, director of the Department of Pediatric Neurology at Qilu Children's Hospital of Shandong University, further introduced that there is about one SMA recessive gene carrier in about every 40-50 people in ordinary people. As an autosomal recessive neuromuscular disorder, genetic probabilities give birth to a child with SMA in every pregnancy if two carriers are combined. Children with infants and young children who develop diseases are most likely not able to live to 2 years old, so SMA is also known as the "number one genetic disease killer" for infants and young children under 2 years old.
The Nanonasen Sodium Injection developed by Bojian is the world's first disease-modifying treatment drug for the cause of SMA. In December 2016, Northinalson sodium injection was approved in the United States. In 2018, Nocinasin sodium injection entered China's "List of the First Batch of Clinically Urgently Needed Overseas New Drugs" and was approved for listing in China in February 2019. At the beginning of the domestic approval for listing, the price of sodium nuocine was 699,700 yuan per unit, which has now been reduced to 550,000 yuan, and the national price is unified.
It is reported that the treatment of sodium northinacin includes two parts: the loading dose and the maintenance dose, which need to be injected in the sheath on the 1st, 14th, 28th and 63rd days, and then every 4 months thereafter, for lifelong medication. According to the latest SMA patient assistance program, patients can enjoy the load dose "1+5" assistance program, that is, buy 1 bottle and get 5 bottles free, and the subsequent maintenance dose assistance program is "1+2". This means that the annual treatment fee for patients is 550,000 yuan.
In the United States, Northinalsan sodium injection is priced at $125,000 per needle, requires six injections in the first year, and costs about $750,000 for treatment, and the cost of the second year has been halved to $375,000. Donna Sullivan, the chief pharmacist at Washington State's Health Care Department, has publicly expressed its displeasure that while the drug gives SMA patients some hope, the huge costs will put enormous pressure on Washington State's Medicaid budget and could lead to lawmakers having to cut back on other optional Medicaid services.
In fact, unlike ordinary drugs, the number of rare disease patients is small, there are few enterprises willing to invest in research and development, and the progress of drug research and development is slow, and in order to recover the cost of research and development, the pricing of enterprises is naturally relatively high.
In addition, during the development process, the failure rate of rare disease drugs is significantly higher. According to a report jointly released by the global biotechnology industry organization BIO, Informa Pharma Intelligence and QLS, in the past decade (2011-2020), 9704 drug clinical development projects have taken an average of 10.5 years from Phase 1 clinical to FDA approval in the United States. Rare disease drugs are four years longer from clinical to approved than other drugs. At the same time, the clinical trials of rare disease drugs also have the characteristics of small sample size, large heterogeneity, and difficulty in recruitment, which makes the failure rate of screening and randomization at any stage of clinical trial significantly higher.
In response to the controversy over drug pricing, Bojian spokesman Matt Fearer has said that this is fully considered. "The pricing strategy of Nocinasine sodium is no different from that of other orphan drugs for rare diseases, and this price is mainly based on the full assessment of the upfront investment of the drug, the clinical value, and the need for Bojian to sustainably invest in other innovative drug research projects."
Jin Enlin, general manager of JD Health Pharmaceutical Department and head of strategy and investment, said: "Rare diseases are first and foremost medical problems, but they are also economic issues. For example, most rare disease patients are still not available, the problem is the failure of the market, how to break the market failure, so that enterprises can produce, develop and provide drugs for a small number of patients, and let these enterprises profit to maintain sustainable development, is a unique challenge in the field of rare diseases. ”
The price reduction rate of this medical insurance negotiation has reached the highest in the past
Negotiations on 94 drugs were successful
The scope of the adjustment of the national medical insurance drug list in 2021 includes drugs newly listed or modified in the past 5 years, national essential drugs and drugs for the treatment of new crown pneumonia. The newly included drugs accurately complement the drug needs of tumors, chronic diseases, anti-infection, rare diseases, women and children, involving a total of 21 clinical groups, patients benefit a wide range, and the accessibility and fairness of mass medication are further improved.
After the adjustment, the total number of drugs in the national medical insurance drug list is 2860, including 1486 western medicines and 1374 chinese proprietary medicines. There are still 892 kinds of Chinese medicine tablets. In the adjustment, the National Medical Security Bureau has always adhered to the functional positioning of "guaranteeing the basics", regarded the affordability of the fund as the "bottom line" that must be adhered to, and focused on meeting the basic drug needs of the majority of insured persons.
From the perspective of price reduction, 94 successful negotiations were held, with an overall success rate of 80.34%. Among them, 67 of the 85 exclusive drugs outside the list were negotiated, with a success rate of 78.82% and an average price reduction of 61.71%. Both in terms of success rate and decline rate, it has also reached the previous highest.
According to 21st Century Business Herald, BeiGene BTK inhibitor Baiyueze (zebutinib) successfully added Fahrenheit macroglobulinemia (WM) indications into the 2021 version of the national medical insurance directory after negotiating the 2021 medical insurance directory, further reducing the economic burden of patients. At present, Baiyueze has three indications included in the medical insurance directory, covering sleeve cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and Fahrenheit macroglobulinemia.
Following the 44% price reduction in the medical insurance negotiations in 2020, Baiyueze has reduced the price by 14% again, and the latest medical insurance implementation price after the second price reduction is 5440 yuan / box, and the monthly treatment cost is 10200 yuan before reimbursement. If calculated according to the national average medical insurance reimbursement ratio of 70%, the monthly treatment fee of Baiyueze after reimbursement will be reduced to about 3060 yuan. After the price reduction, Baiyueze became the product with the lowest monthly treatment cost among similar BTK inhibitors.
The controversial Green Valley Pharmaceuticals' Alzheimer's drug Phase 9 (Mannit Sodium) was reduced by 66% into the medical insurance directory, and the latest price was 7.05 yuan / 150mg.
With the continuous deepening of medical insurance reform, the landing time of medical insurance policies is also shortening year by year. From the announcement of the results of the negotiations to the time the patients receive treatment under the Medicare reimbursement policy, it takes less than a month. It can be seen that while ensuring the efficient implementation of the policy, it has become a trend to truly open up the "last mile" of medical insurance drugs for patients.
IQVIA also believes that from the results of this medical insurance negotiation, policy changes have guided the innovation and upgrading of the pharmaceutical industry, and the commercial success of drugs in the future will rely more on innovation. On the one hand, policy changes have brought about the advance of the time point of listing and medical insurance access, and the key points of drug commercial success have been advanced; on the other hand, centralized procurement has led to the shortening of the drug life cycle, and enterprises need to innovate and win; at the same time, the commercial success of drugs will gradually transform from relying on sales to relying on evidence production, value positioning and value transmission; in addition, measures such as dynamic adjustment and collection of medical insurance catalogues have forced the pharmaceutical industry to innovate and upgrade while optimizing the use of funds.