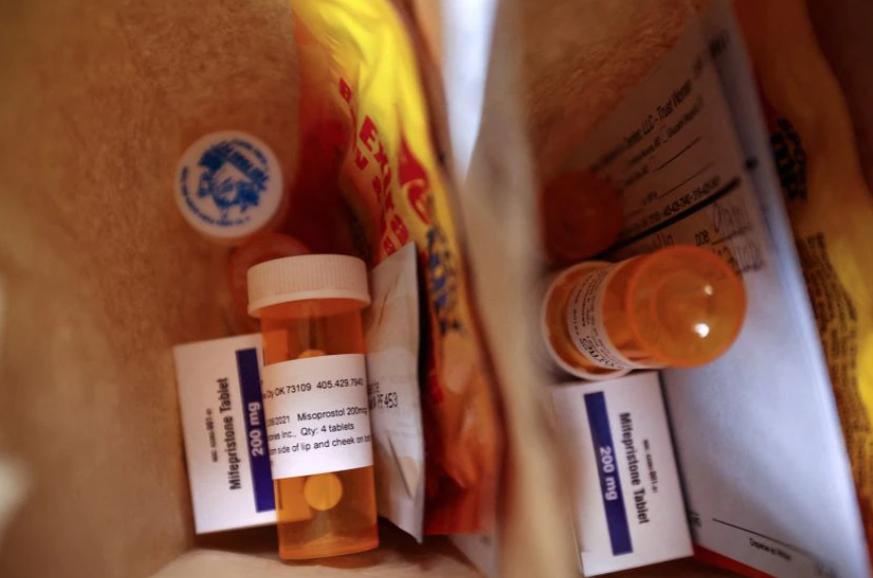
The Comprehensive Associated Press and Reuters reported on the 17th that the US Food and Drug Administration (FDA) announced on the 16th that it has permanently relaxed the key restrictions on a drug used to terminate early pregnancy - mifepristone, canceling the long-standing requirement of personally taking the drug. Millions of U.S. women now have access to prescriptions through online counseling and medications by mail.
Source: Reuters
The FDA eases restrictions on mifepristone
Mifepristone is a steroid-type antiprogestin preparation that is used sequentially with prostaglandin drugs and can be used to terminate pregnancy within 10 weeks.
Restrictions on the drug have been in place since the FDA approved it in 2000. Earlier this year, due to the COVID-19 pandemic, the U.S. government temporarily lifted restrictions on the drug. Women are able to consult with a healthcare provider through telemedicine and receive the pill by mail. The FDA's decision of 16 makes this temporary change permanent.
In the United States, about 40 percent of abortions are done through medication rather than surgery. This option has become even more critical during the COVID-19 pandemic.
Due to changes in FDA rules, many women will not need to go to the clinic or hospital in person to get the drug, and can choose to get a prescription for an online consultation and get the drug from the pharmacy by mail. This decision will increase the chances of women in remote and rural areas to receive medical abortions when they face obstacles such as inaccessibility and leave.
However, 19 states, including Texas, have replaced the FDA's decision with laws banning telehealth consultations or mailing abortion pills, but women in those states may travel to other states for medical abortions.
Some restrictions are still retained
FDA records show that between September 2000 and December 2018, 24 of the 3.7 million women who took mifepristone to terminate their pregnancies died of complications. But because of underlying health conditions and other factors, not all deaths can be directly attributed to the drug. Common side effects of the drug include cramps, bleeding, nausea, headaches, and diarrhea. In some cases, surgery may be required to stop the bleeding.
The FDA still retains restrictions, such as the fact that the drug must be sold through a certified pharmacy and a certified prescribing physician.
The move sparked controversy
The FDA's decision is the latest shift in the polarized legal battle over medical abortion, which will only intensify in the context of COVID-19. That would certainly trigger legal challenges and more restrictions in Republican-dominated states.
Georgina Usova, an attorney for the Acliberties Union, said: "The FDA's decision will greatly alleviate the suffering of countless miscarriage patients. "However, it is disappointing that the FDA has failed to repeal all medically unnecessary restrictions on mifepristone, and these remaining barriers should also be removed."
Jenny Mansini, president of the American Foundation for the Education and Protection of Life, said the decision "will result in more deaths from abortion and will increase the number of mothers who have suffered physical and psychological harm as a result of drug abortion." ”
Upstream News Compiled by Ruochen Yang