The small editor of the cold-resistant plasticizer manufacturer today takes you to know about the determination of our ethyl acetate saponification reaction rate constant, and the determination of the saponification rate constant of ethyl acetate by conductivity is the basic experiment of the physicochemical kinetics part. Currently, most universities conduct saponification experiments under conditions where the initial concentrations of ethyl acetate and sodium hydroxide are equal. However, ethyl acetate is volatile, and its aqueous solution will be hydrolyzed. Sodium hydroxide must also be reconstituted, which is difficult to satisfy. At the same time, the experiment requires a constant temperature and a short reactor mixing time, which makes it difficult to find a suitable reactor.
In view of the problems existing in the experiment, some improvement measures were taken, mainly including two aspects: (1) the reactor was improved; (2) the mixture method of the reactants was improved, and after the improvement, the students' experiments had a good effect.
Cold-resistant plasticizer manufacturers introduce the determination of the rate constant of the saponification reaction of ethyl acetate
1. Experimental instruments and reagents
Instruments: saponification reaction experimental device, electronic balance (specification 1200g/0.1g), electronic balance (specification 110g/0.1mg), pure water machine (UPT-I-20T), single-channel pipette (specification 10-100μL), magnetic stirrer (78HW-1), timer, thermostatic reactor (homemade), alkaline burette (50.00mL).
Reagents: sodium hydroxide (analytically pure), ethyl acetate (analytically pure), potassium hydrogen phthalate (analytically pure), pure water, phenolphthalein indicator.
2. Experimental principle
Detailed experimental principles can be found in the textbook, this experiment uses the following rate equation to calculate the rate constant:
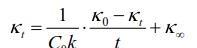
By experimentally measuring the conductivity of the starting solution κ0 and the conductivity of the t solution at different times, the kat pair (κ0-κt)/t is plotted, and a straight line is obtained, and the reaction rate number k value can be obtained from the slope of the line. Experimental data are processed using Origin software to obtain the slope of the line and the correlation coefficient R.
3. Experimental steps
The experimental operation steps are basically the same as the textbook, and the improved operations are listed below.
(1) Experimental apparatus diagram
Improved experimental apparatus: the reactor is in the shape of a high-profile beaker inside, and the outside is a thermostatic jacket; In order to make the solution inside the reactor have enough height and the cup mouth to have enough width, to facilitate the magnet stirring and placement of conductivity electrodes and thermometer, the reactor interior is designed as trapezoidal. The cup mouth is sealed with a silicone (or rubber) plug and 3 round holes are opened, which are the electrode socket, the thermometer socket and the dosing port. Improved experimental apparatus: the reactor is in the shape of a high beaker on the inside and a thermostatic jacket on the outside. To make the solution in the reactor high enough, the cup wide enough to stir the magnet and place the conductive electrode and the thermometer, the reactor is designed internally as a trapezoid. The cup mouth is sealed with a silicone (or rubber) plug and three round holes are opened, namely the electrode holder, the thermometer holder and the sample holder.
(2) Solution preparation
The sodium hydroxide solids are weighed with an accuracy of 0.1 g balance, and a certain volume of solution is formulated with fresh pure water at a concentration of about 0.01 mol/L. Potassium hydrogen phthalate was accurately weighed with an accuracy 0.1 mg balance and the sodium hydroxide solution was calibrated. After obtaining the accurate concentration C0, calculate the volume of pure ethyl acetate V (μL) using the following formula to calculate an equal molar amount:
where C0 and V0 are the concentration and volume of NaOH solution, respectively;
M1, ρ1 and w1 analyzed the molecular weight, density and mass fractions of pure ethyl acetate, respectively.
(3) Determination of κ0 and κt
1. Connect the constant temperature water bath with a latex tube, open the constant temperature water bath, and set the temperature.
2. Accurately take 50.00mL sodium hydroxide solution with a belly pipette and place it in the reactor, slowly stir the magnetic stirrer, and after the temperature is constant, the conductivity is determined κ0.
3. After continuous rapid stirring for about 1min, slow down the stirring speed and keep the slow speed and stir evenly. Then record the conductivity of the 2, 4, 6, 8, 10, 12, 15, 20, 15, 20, 25, 30, 35, 40min moments in turn.
4. Calculate the amount of ethyl acetate required, and take the amount with a pipette with a measuring range of 10~100 μL.
5. Clean experimental supplies and use Origin software to process experimental data.
6. Turn the speed of the magnetic stirrer to Z, remove the rubber stopper and add ethyl acetate, time it at the same time, and then plug the rubber stopper.
The small editor of the cold-resistant plasticizer manufacturer will introduce you to here today, and those who have questions can consult us by phone!
Article source: https://www.honyansz.cn/m