According to the news on the official website of NMPA on March 24, the bivalent (type 16/18) vaccine of recombinant human papillomavirus (type 16/18) of Yuxi Zerun Biology, a subsidiary of Watson Biology, was approved for marketing.
The bivalent HPV vaccine developed by Shanghai Zerun is used to prevent cervical cancer caused by HPV16 and 18 virus infection. At present, hpvic vaccines that have been marketed worldwide include the divalent HPV vaccine of GlaxoSmithKline (GSK) in the United Kingdom, the quadrivalent HPV vaccine and the nine-valent HPV vaccine of Merck Inc. in the United States, and the divalent HPV vaccine of Xiamen Wantai Canghai Biotechnology Co., Ltd. in the mainland.
The hpvic vaccine that will be approved this time is produced by the Pichi yeast expression system, which has many advantages, such as the high expression level of the yeast, which is conducive to isolation and purification; the expression can be strictly controlled; and the stability of the expression product is high. In addition to the bivalent HPV vaccine, Runze Bio's product pipeline also includes nine-valent HPV vaccine, recombinant enterovirus type 71 (EV71) virus-like granule vaccine, and new coronavirus vaccine.
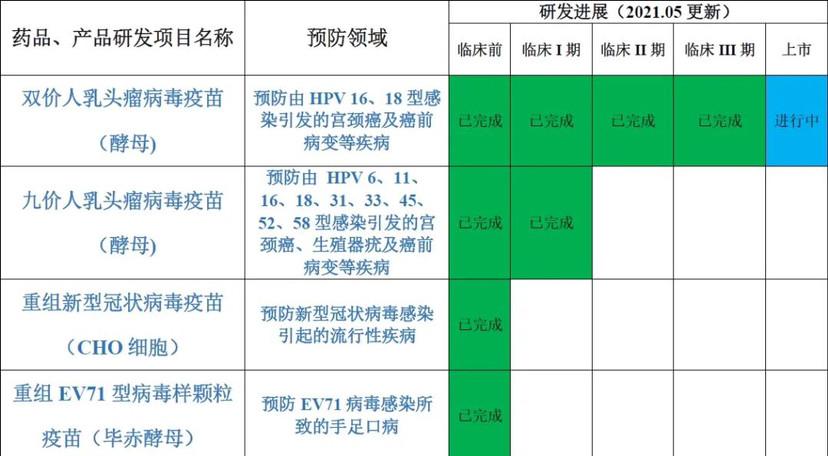
According to the official website of Runze Biology
At present, there are 4 HPV vaccines on the market in China, namely Merck's 4-valent and 9-valent vaccines, GSK's 2-valent vaccines, and Wantai Bio's 2-valent vaccines (trade name: Xinkenin). It is reported that in 2020, the sales volume of Xinkoning in China is about RMB693 million. In 2021, GSK's bivalent vaccine sales were $138 million, and Merck's quadrivalent and nine-valent vaccines (Gardasil/Gardasil 9) were $5.673 billion, up 44% year-over-year. (Liu Yingqi)