What are some of the new lung cancer targeted drugs in 2021? Let's take a look!
In 2021, under the guidance of the concept of "precision medicine", a number of new drugs targeting rare targets in the field of lung cancer were officially approved to enter the clinic, benefiting patients with rare mutations with relatively limited treatment methods. In addition, more and more new drugs/new indications for already relatively mature lung cancer targets such as EGFR are being marketed, bringing more choices to clinicians and patients.
What are the new drugs/indications for targeted therapies for heavy lung cancer approved in China and the United States in 2021? The "Medical Oncology Channel" is organized below for the benefit of readers.
Pratinifib
On March 23, 2021, CStone's RET inhibitor pratinib was approved by the State Drug Administration (NMPA) for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who had previously received platinum-containing chemotherapy positive for RET gene fusion, becoming the first approved RET inhibitor in China. Adults with locally advanced or metastatic NSCLC who have previously received platinum-containing chemotherapy with positive RET gene fusion.
The incidence of RET gene fusion in patients with NSCLC in mainland China is about 1.4% to 2.5%[1]. Pratinib's approval in China is based on Chinese patient data from the global Phase I/II ARROW study published at the 2020 World Lung Cancer Congress (WCLC). As of May 22, 2020, the ARROW study included a total of 37 patients with advanced RET fusion positive NSCLC from 10 research centers in China. Results showed that the confirmed objective response rate (ORR) was 56 percent (95 percent CI: 38 to 74 percent) in 32 of these patients with assessable lesions, with one achieving complete remission and 17 achieving partial response, with a disease control rate of 97 percent, consistent with global results, and good safety and tolerability in the Chinese patient population [2].
Figure 1: Efficacy results of pratinib Chinese group
Oscitinib
On April 14, 2021, AstraZeneca's third-generation EGFR-TKI osimtinib added a new indication, approved by NMPA, for adjuvant therapy after tumor resection in adult patients with EGFR-sensitive mutations in NSCLC.
The approval of this indication is based on the ADAURA study led by Professor Wu Yilong of Guangdong Provincial People's Hospital. The results of the ADAURA study showed that in patients with NSCLC stage II-IIIB (T3N2, AJCC8), adjuvant therapy with osimertinib significantly prolonged the median disease-free survival of patients (DFS; not reaching vs. 19.6 months; HR 0.17; P<0.001). DFS rates were significantly better in the 1-, 2-, and 3-year DFS rates in patients treated with osimtinib than in the placebo group (97 percent vs. 61 percent, 90 percent vs. 44 percent, 80 percent vs. 28 percent) [3].
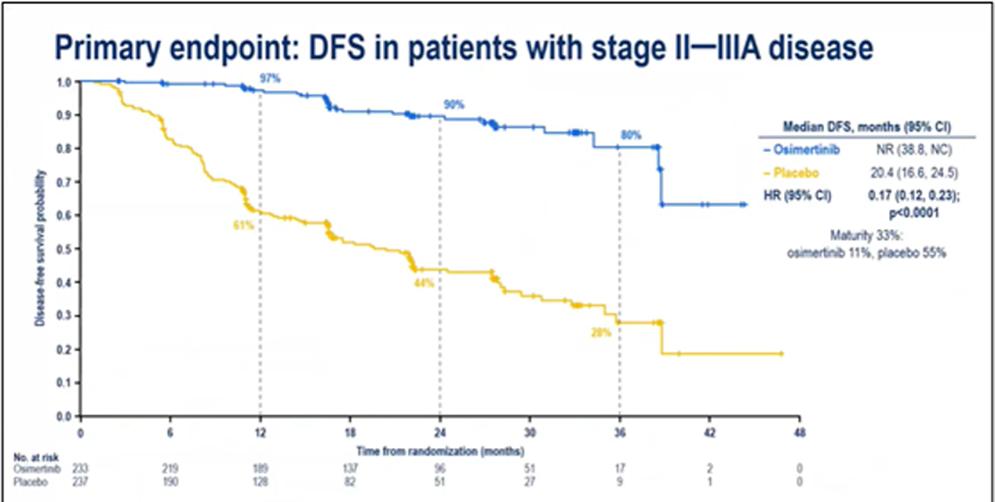
Figure 2: Results of the primary endpoints of the ADAURA study
In addition, data from the ADAURA study subgroup published by the 2020 WCLC show that adjuvant therapy with osimtinib still significantly improves DFS (HR 0.23) in patients who have not received adjuvant chemotherapy prior to adjuvant therapy with osimtinib [4].
Exetinib
On June 3, 2021, Beta Pharmaceutical's extinib was approved by NMPA for postoperative adjuvant therapy of stage II.-III.A EGFR mutation NSCLC.
The approval of the indications for exetinib adjuvant therapy is based on a phase III multicenter, randomized controlled EVIDENCE study. The study enrolled patients with NSCLC mutations with stage II-IIIA EGFR mutations who received exetinib treatment or standard adjuvant chemotherapy.
The results showed that the median DFS in the exetinib treatment group and the standard adjuvant chemotherapy group were 46.95 months and 22.11 months, respectively (HR 0.36; 95% CI: 0.24-0.55, p<0.0001). The three-year DFS rates of 63.88% and 32.47% in the exetinib adjuvant therapy group and the standard adjuvant chemotherapy group were not available, respectively, and five-year DFS data and overall survival (OS) data were not available [5].
Figure 3: EVIDENCE studies DFS data
Severatinib
On June 22, 2021, the MET inhibitor sivatinib, developed by AstraZeneca and Huang Pharmaceuticals, was approved by NMPA for the treatment of locally advanced or metastatic NSCLC of exon-hopping mutations in MET 14.
MET 14 exon-jump mutation is an independent oncogenic driver gene mutation that occurs in approximately 3 to 4 percent of NSCLC patients, and can occur in lung sarcoma-like carcinomas as high as 31.8 percent [6]. The approval of severatinib in China is based on a Phase II study in China led by Professor Lu Shun of the Chest Hospital affiliated to Shanghai Jiao Tong University, which was published in The Lancet-Respiratory Medicine in June 2021.
As of the data analysis deadline (3 August 2020), the Independent Review Committee (IRC) conducted a statistical analysis of 61 patients with assessable efficacy, with a Sivatinib treatment orr of 49.2% (95% CI: 36.1%-62.3%), and a disease control rate (DCR) of 93.4% (95% CI: 84.1%-98.2%). In the full assay set of patients (70 patients), the median progression-free survival (PFS) of sivotinib treatment assessed by IRC was 6.8 months. In addition, cevotinib was well controlled by brain metastases, with 15 patients with baseline brain metastases shrinking or remaining stable after cevotinib treatment [7].
The safety profile of cevotinib treatment is good, most adverse events (AE) are grade 1-2, which can be resolved by adjusting the dose or discontinuing the drug, and patients have not developed interstitial pneumonia.
Figure 4: Optimal change in target lesions in all patients in the sivotinib study from baseline
Sotorasib(AMG-510)
In NSCLC patients, the proportion of positive KRAS mutations is higher than that of targets such as RET and ROS1, so there is considerable clinical attention to targeted therapy targeting KRAS. Despite this, the current breakthrough in targeted therapy for this target is still relatively limited.
On May 28, 2021, Sotorasib (AMG-510) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with locally advanced or metastatic NSCLC with KRAS G12C mutations who had previously undergone at least one systemic therapy.
Approval of AMG-510 is based on the results of the largest phase II CodeBreaK-100 study conducted to date in patients with KRAS G12C mutations in the late-stage NSCLC cohort. 124 patients with KRAS G12C mutation-positive NSCLC who had progressed after immunotherapy and/or chemotherapy received AMG-510 (960 mg orally once daily). Results showed that 36 percent (95 percent CI: 28 percent to 45 percent) of patients treated with AMG-510, 81 percent (95 percent CI: 73 percent to 87 percent) achieved disease control (complete remission, partial response, and stabilization for more than three months), and median DoR was 10 months [8].
The most common adverse reactions in patients were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough (20% of ≥). Treatment was discontinued in 9% of patients due to adverse reactions.
bibliography
1. Expert Consensus on Clinical Detection of RET Gene Fusion in Non-Small Cell Lung Cancer in China. Chinese Journal of Pathology, 2021, 50(6): 583-591.
2.Q Zhou, et al. WCLC 2020. FP14.17.
3.YL Wu, et al. NEngl J Med, 2020, 383: 1711-1723.
4.YL Wu, et al. WCLC 2020.
5.Zhou CC, et al. WCLC 2020, abstract FP14.11.
6.Tong JH, et al. Clin Cancer Res. 2016 Jun 15;22(12):3048-56.
7.Shun Lu, et al. Lancet Respir Med. 2021 Jun 21; S2213-2600(21)00084-9.
8.CodeBreaK 100. 2020WCLC. PS01.07.
*Materials are supported by AstraZeneca and are intended for healthcare professionals only
Approval number: CN-91191 Expiration date: 2023-2-25
*The copyright of this article belongs to the original author, if you need to reprint, please contact the original author for authorization