The 2021 ESMO Conference has contributed a number of blockbuster research results to us. With the third generation of EGFR-TKI entering the clinic, the targeted therapy of EGFR mutation-positive NSCLC patients has blossomed in many areas of advanced and early adjuvant, so how should the adjuvant targeted therapy time of EGFR mutation-positive NSCLC patients be determined? In this issue, we will explore the choice of adjuvant targeted therapy timing.
From late to early
The exploration of adjuvant therapy from chemotherapy to immunotherapy and precision-targeted therapy. About 30% of patients with resectable NSCLC have postoperative adjuvant chemotherapy, which is ineffective and significantly affects the prognosis of patients1, and the significant improvement in the efficacy of various EGFR-TKIs, especially third-generation TKIs, provides the possibility of adjuvant targeted therapy for patients with EGFR mutation-positive NSCLC. However, early clinical exploration was not smooth sailing, until the results of the 2020 Phase III clinical trial ADAURA showed that adjuvant therapy with osimtinib reduced the risk of disease recurrence or death by 80%2, and adjuvant therapy based on molecular targeting became more and more important.
First-generation EGFR-TKI targeted adjuvant therapy,
Is 2 years enough?
Previous data show that the median recurrence time of phase II. to III. NSCLC is 9 to 21 months, the recurrence of N1 is about 21 months, and the recurrence of N2 stage is 3-6 in 9 to 10 months.
To reduce recurrence, the duration of a generation of EGFR-TKI adjuvant therapy is usually designed to exceed 2 years of median DFS. For example, RADIANT, SELECT, and EVAN are all adjuvant targeted therapies for erlotinib for 2 years, and the targeted therapy time for EVIDENCE to study exetinib is also 2 years 3-6. However, a generation of EGFR TKI adjuvant targeted therapy studies have shown that the risk of recurrence increases again after 2 years of adjuvant targeted therapy, with the highest recurrence rate occurring around the 6th month after discontinuation7. This also raises our questions: EGFR-TKI adjuvant targeted therapy, 2 years is enough?
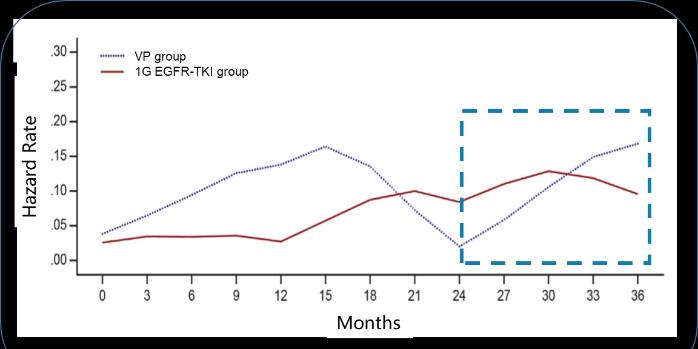
Longer duration of administration may have a better survival benefit
ICOMPARE Study 8 was the first to compare the effect of treatment duration on efficacy of exetinib. The Phase II clinical study included a total of 109 patients, those treated with 1-year and 2-year ektinib, and as of 24 August 2020 (data deadline), the median follow-up time was 44.1 months, and the median DFS was 48.92 months and 32.89 months, respectively, with a statistically significant difference (HR=0.521, P=0.039), and the DFS benefit was better in the 2-year group than the 1-year group. Although the secondary endpoints were not yet mature, the difference was statistically significant (HR =0.344, P=0.032), and the 2-year group still had a longer OS than the 1-year group, with a 6-year OS rate of 51.3% in the 1-year group and 74.6% in the 2-year group.
EGFR-TKI targeted adjuvant therapy had a significant benefit over 3 years
So what is the survival benefit of EGFR-TKI adjuvant therapy for 3 years? The ADAURA study explored the third generation of EGFR TKI adjuvant targeted therapy studies designed for 3 years2. Another third-generation EGFR TKI adjuvant targeted therapy study also designed the time to be 3 years. The results of the study are highly anticipated.
In both trials, the ADAURA study was prematurely blinded due to its efficacy, by which time the study had been enrolled and all patients had been followed up for at least 1 year. Of the 470 patients with stage II-IIIA disease, the osimertinib group did not achieve median disease-free survival, compared with 19.6 months in the placebo group and 0.17 hrs. Of the 682 patients in the overall 682 stage IB-IIIA patients, the osimtinib group did not achieve median disease-free survival, compared with 27.5 months in the placebo group and 0.202 hr. In summary, in patients with positive NSCLC mutations in IB to IIIA, patients receiving osimertinib had significantly longer disease-free survival than those receiving placebo.
brief summary
Summarizing today's topic, we found that the duration of the past generation of EGFR TKI adjuvant targeted therapy studies was usually 2 years 3-6, but prolonging the duration of EGFR-TKI adjuvant therapy may have a better survival benefit, and the current three-generation EGFR TKI adjuvant therapy study is designed for 3 years, of which the ADAURA study results show that adjuvant osimertinib treatment for 3 years can reduce the risk of disease recurrence and death2. How is the optimal duration of administration for adjuvant targeted therapy determined? What are the key determinants deserve more exploration, and more trial evidence is needed to guide clinical applications in the future.
bibliography:
1.Pignon J-P, et al. J Clin Oncol 2008;26:3552-92. Wu YL, et al. N Engl J Med. 2020 Oct 29;383(18):1711-1723
3.Kelly K, et al. J Clin Oncol 2015;33:4007–4014
4.Pennell NA, et al. J Clin Oncol 2019;37:97–104
5.Yue D, et al. Lancet Respir Med 2018;6:863–873
6.Zhou CC, et al. Lancet Respir Med 2021;9:1021–1029
7.Zhong WZ, et al. Lancet Oncol 2018;19:139–148.
8.Dongsheng Yue, et al.2021 ASCO. Abstract # 8520
*This information is for medical and scientific exchange by medical and health professionals only and is not intended for promotional purposes.
Approval number: CN-88318 Expiration date: 2022-11-16
Note: ESMO, European Society of Oncology; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; EGFR, human epidermal growth factor receptor; NSCLC, non-small cell lung cancer; OS, overall survival
*The copyright of this article belongs to the original author, if you need to reprint, please contact the original author for authorization