Health Times reporter Qiu Yue
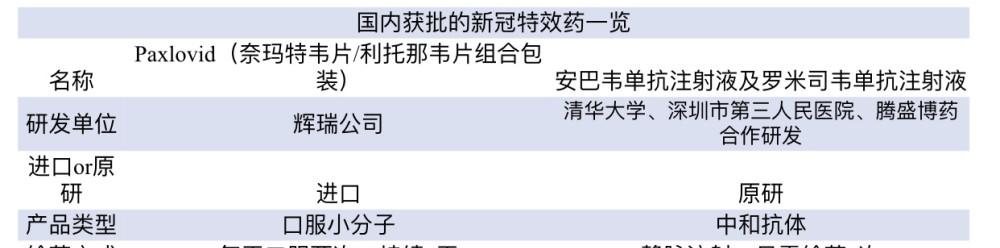
On February 11, the State Food and Drug Administration conditionally approved the import registration of Pfizer's new coronavirus treatment drug nematvir tablets/ritonavir tablets combination packaging (i.e. Paxlovid). This is the first oral COVID-19 drug listed in China, and the second new crown treatment drug listed in China so far.
On December 8, 2021, the State Drug Administration (NMPA) approved the registration application of Tengsheng Huachuang Pharmaceutical Technology (Beijing) Co., Ltd. for the combination treatment of new coronavirus neutralization antibody combination therapy drugs ampavirine monoclonal antibody injection (BRII-196) and romisvir monoclonal antibody injection (BRII-198).
When can domestic patients use the new crown special drug? On February 14, a Health Times reporter contacted Pfizer and Tengsheng Bo Pharmaceutical respectively.
Draft: Qiu Yue
The first oral NEW CROWN drug in China: the listing news will be announced in a timely manner
According to the State Food and Drug Administration, Paxlovid is an oral small molecule new coronavirus treatment drug for the treatment of mild to moderate novel coronavirus pneumonia (COVID-19) in adults with a high risk factor for progression to severe disease, such as patients with severe risk factors such as advanced age, chronic kidney disease, diabetes, cardiovascular disease, and chronic lung disease.
On February 14, the Health Times reporter learned from Pfizer that Paxlovid has just been approved, the specific listing plan is not yet under control, if you want to know when the drug will be listed, in what city which hospitals landed and other information, it will take some time. There will be news to be announced as soon as possible.
Image courtesy of Xinhua News Agency.
According to the U.S. Food and Drug Administration (FDA), Paxlovid is composed of nirmatrelvir tablets and ritonavir tablets, which inhibit the replication of the SARS-CoV-2 protein, while ritonavir tablets can slow the breakdown of nematvir tablets and help drugs stay in the body for longer periods of time at higher concentrations. Treatment is followed by three pills, namely two nematvir tablets and one ritonavir tablet, taken orally twice daily for 5 days and not for more than 5 consecutive days.
The U.S. Food and Drug Administration stressed in a statement that Paxlovid is not a substitute for vaccination for individuals who recommend COVID-19 vaccines and booster doses.
The number of clinical trials showed an 89% lower risk of COVID-19-related hospitalization or death from any cause in adults treated with Paxlovid within three days of symptom onset (primary endpoint) compared to placebo. In patients treated within 5 days of the onset of symptoms (secondary endpoint), the risk of hospitalization or death for any cause was reduced by 88%, and no death was observed in the treatment group.
The first domestic NEW CROWN monoclonal neutralizing antibody: the production and supply of products are being promoted
On December 8, 2021, the State Drug Administration (NMPA) approved the registration application of Tengsheng Huachuang Pharmaceutical Technology (Beijing) Co., Ltd. for the combination treatment of new coronavirus neutralization antibody combination therapy drugs ambavir monoclonal antibody injection (BRII-196) and romimavir monoclonal antibody injection (BRII-198), and approved the combination of the above two drugs for the treatment of mild and ordinary types and accompanied by high-risk factors (12-17 years old) that progress to severe (including hospitalization or death), People with ≥40 kg) of covid-19 infection. Among them, adolescents (12-17 years old, weight ≥40kg) are conditionally approved.
On February 14, a Health Times reporter learned from Tengsheng Huachuang that the company is actively promoting production and supply work to achieve drug marketing as soon as possible to benefit new crown patients.
Ambavir maclizumab and romizumab are the first approved drugs with independent intellectual property rights in mainland China for the combined treatment of new coronavirus neutralizing antibodies, which are jointly developed by Tsinghua University, Shenzhen Third People's Hospital and Tengsheng Bo Pharmaceutical Biotechnology Company.
Courtesy of the enterprise.
On December 4, 2021, Tengsheng Huachuang obtained data from all subjects in the Phase III clinical trial. The final results showed that the combination therapy reduced the risk of hospitalization and death in clinically high-risk COVID-19 outpatients by 80% compared with placebo, and the clinical safety of the drug was better than that of the placebo group. The current in vitro chimeric virus detection data show that the combination therapy has maintained neutralization activity on the new coronavirus variants such as Omicron, Delta, Delta+, Alpha, Beta, Gamma, ramda and so on.
Zhang Linqi, a professor at Tsinghua University School of Medicine, said at the product launch that whether subjects who started treatment early (within 5 days after symptoms appeared) or late stages (within 6 to 10 days after symptoms appeared), hospitalization and mortality were significantly reduced, which provided a longer treatment window for new crown patients.
According to the information provided by Tengsheng Bo Medicine, the new crown virus mainly infects human cells by binding to ACE2 on human cells, and causes diseases through cell reproduction. Ampavitomab blocks the binding of the virus to ACE2 at the site where the new coronavirus receptor binds directly to ACE2. Romizumil monoclonal antibody targets another site in the receptor binding region of the new coronavirus that does not bind to ACE2, blocking the reproduction of the virus with different mechanisms of action. It is reported that the neutralizing antibody combination therapy adopts intravenous injection, an injection time of about 1 hour, and only one dose is needed to complete the treatment.
In addition, the Guangzhou laboratory team led by Academician Zhong Nanshan is carrying out research on the use of combined therapy between amphavir monoclonal antibody and romizumab for prevention, and promoting their preventive use in people with poor vaccine response.