As a new target of lung cancer, MET has broken the treatment deadlock in the past two years, and in 2020, it has ushered in two targeted drugs and quickly incorporated into the treatment guidelines, bringing more choices and hopes for the survival of patients with advanced cancer.
At the beginning of 2022, the FDA once again received good news by awarding a new antibody-conjugated drug telisotuzumab vedotin breakthrough therapy designation for the treatment of patients with c-Met overexpression of EGFR wild-type, non-squamous non-small cell lung cancer that progresses in advanced or metastatic platinum therapy.
More than half of patients have objective remission! The FDA granted ABBV-399 breakthrough treatment designation
The FDA's award of Ttelisotuzumab vedotin (AbbVie, codenamed ABBV-399) as a breakthrough treatment designation is based on the excellent data from its Phase 2 LUMINOSITY study.
As of December 2018, a total of 42 patients were included in the trial's expanded cohort. Of these patients, 37 were c-Met positive. It is worth mentioning that most of these patients are advanced patients who are resistant and progress after receiving multiple treatment regimens.
The results showed that the objective response rate (ORR) was 53.8% in patients with high c-Met expression, 25% in patients with moderate c-Met expression, and 34.5% and 86.2% in patients with dual mutations of c-Met/EGFR.
There are also drugs approved for the treatment of C-MET overexpression in non-small cell lung cancer, and ABBV-399 brings new hope to this group of patients.
Related reading: MET+ lung cancer patients usher in spring! Five new drugs break the treatment deadlock
About ABBV-399
Telisotuzumab Vedotin (Teliso-v, ABBV-399) is a novel c-MET target antibody drug conjugate (ADC) with excellent potential in the treatment of patients with non-small cell lung cancer overexpressed c-MET.
Drug Name: Telisotuzumab Vedotin (ABBV-399)
Target: MET protein overexpression
R&D company: AbbVie
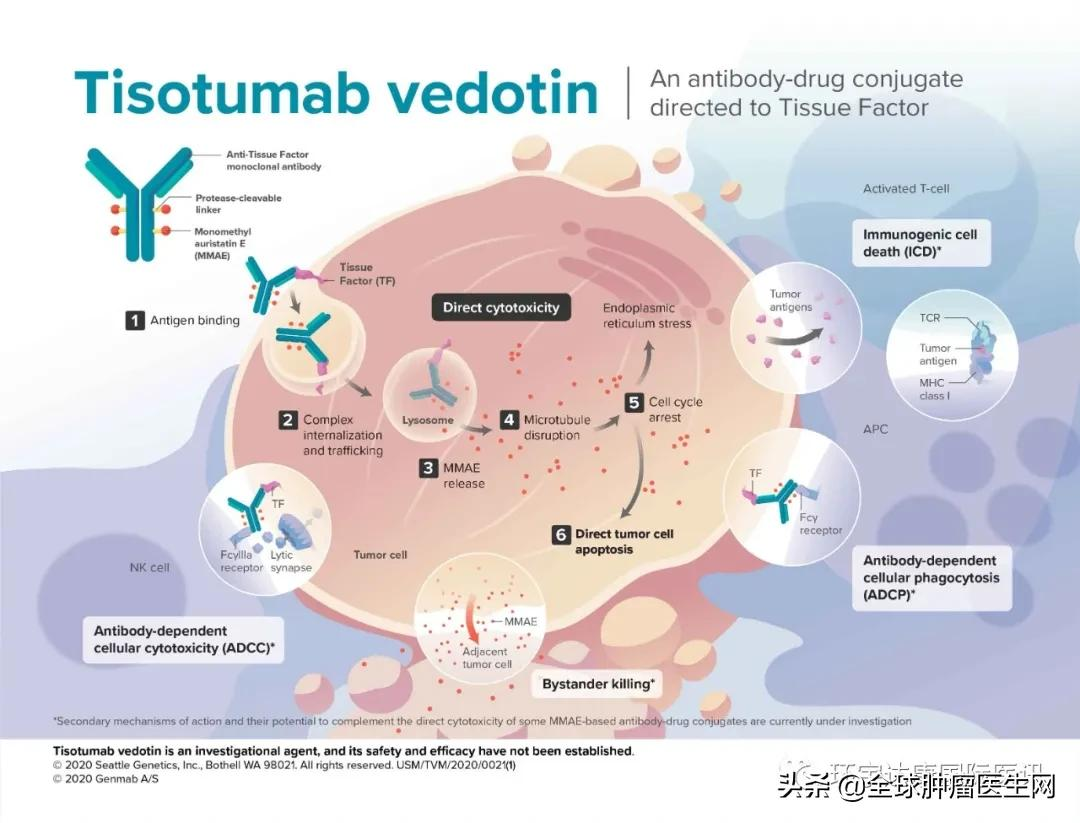
The full name of the MET gene is mesenchymal-epithelial transition factor (MET). C-MET is a hepatocyte growth factor (HGF) receptor involved in cell survival and proliferation.
In non-small cell lung cancer, the most common is exon 14 jump mutation, which occurs in 3-4%, and in addition, THE EXPANSION OF MET produced by EGFR inhibitor resistance is also very common, accounting for about 5% to 20% of the EGFR-TKI resistance mechanism.
What requires special attention from lung cancer patients is that patients with MET mutations respond poorly to immunotherapy, even if the expression of PD-L1 is high .
MET gene abnormalities play an important role in the occurrence and development of NSCLC, drug resistance, and prognosis, and this gene target is also one of the top ten targets recommended for patient detection in the NCN guidelines. Studies recommend that all lung cancer patients should have a MET test.
The good news is that this new antibody-coupled drug is currently undergoing clinical trials in China, and it needs to be reminded that even if the standard treatment plan fails, genetic testing can be tried, and once there is a relevant genetic mutation, these specific anti-cancer drugs can be tried.