On the 22nd, according to the official website of Chengdu Wesker Biopharmaceutical Co., Ltd. (hereinafter referred to as "Wescold Bio"), recently, under the funding of the national emergency research project for the mutant new coronavirus vaccine, the second-generation new crown vaccine of Wescolm has made important progress against the Omicron (Omicron) variant.
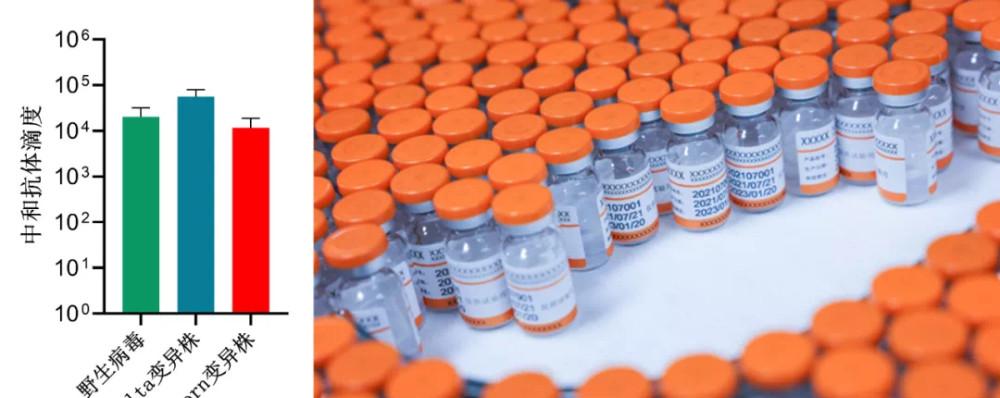
▲Second-generation COVID-19 vaccines target wild viruses and neutralizing antibody titers of different variants
It is understood that the vaccine belongs to the latest fifth-generation vaccine technology (fully synthetic vaccine), for the S protein of the new crown mutant strain, based on the structure of the precise design of subunit vaccine antigens, can be self-assembled into a stable protein granular vaccine. The large-scale production of vaccines adopts internationally advanced insect cell production technology and cooperates with international new vaccine adjuvants. After immunizing mice or monkeys and other animals, the vaccine can induce the production of high-titer neutralizing antibodies, and the neutralizing antibodies against the Omicron variant can reach tens of thousands of levels (representing the ability to block virus-infected cells with 10,000 times blood dilution), and it has also been found that the vaccine has completely inhibited the neutralizing antibodies of the true virus of various mutant strains such as the Brazilian strain, the South African strain, and the Delta strain, which indicates that the vaccine is a universal new crown vaccine for a variety of variant strains. It is also the first COVID-19 vaccine with high titer neutralizing antibodies to the Omicron variant released internationally.
At present, the vaccine has completed process research and large-scale preparation, the production process is stable, the product has been qualified by the China Institute of Drug and Food Testing; the pharmacodynamic research and pre-clinical safety evaluation of the immunogenicity and protective power of mice, rats and monkeys have been completed; and the declaration materials have been submitted to the State Food and Drug Administration on a rolling basis in August this year, and it is expected to officially enter clinical trials in early 2022.
Neutralizing antibodies represent the ability of vaccines to produce antibodies to neutralize viruses, and are an important indicator for judging the effectiveness of vaccines in the world. According to the latest article published in the journal Science, data from multiple clinical studies show that neutralizing antibody titers directly determine vaccine protection: vaccines induce the production of neutralizing antibody titers of NT50 of 10, 100 and 1000, corresponding to 78%, 91% and 96% of protective power after vaccination. Wesker Bio's second-generation COVID-19 vaccine has a neutralizing antibody NT50 for multiple variants, which can reach tens of thousands, and it is reasonable to speculate that the vaccine is one of the most powerful weapons against the new crown virus of various variants.
Chengdu Wesker Biomedical Co., Ltd. was founded in July 2020 by more than 10 scientists from the State Key Laboratory of Biotherapy in West China Hospital of Sichuan University under the leadership of Academician Wei Yuquan, and was co-founded by the school, a team of scientists and Chengdu High-tech Zone. So far, the company has carried out series A and B financing, and completed more than 2 billion yuan of financing.
At present, Wescotec Has built a production base with an annual production capacity of 100 million doses in Chengdu Tianfu International Bio-City, has completed the construction and verification of the GMP production workshop, and obtained the "Drug Production License" issued by the Sichuan Provincial Food and Drug Administration. The company has a mature insect cell expression platform, mRNA vaccine platform, new adjuvant platform, bacterial vaccine platform and tumor vaccine and immunotherapy platform, with 21 pipelines such as new crown vaccine, multivalent influenza vaccine, herpes virus vaccine, tumor immunopharm preparations, etc., of which the phase 3 clinical trial of a generation of new crown vaccine has been completed and clinical trials have been completed in Japan.
Red Star News reporter Peng Xiangping pictured according to the official website of Wesker Biology
Edited by Yixi Chen
(Download Red Star News, there are prizes for the newspaper!) )