Critical states are widespread in complex systems and are characterized by small changes that can cause large avalanche changes. Nature Communications' July paper, "Designing Self-Tissue Critical States in Living Cells," reproduced the critical state for the first time in synthetic biology using two genes in E. coli to regulate each other.
1. Self-organizing criticality is no stranger
In the summer, children play on the beach, often piling up larger and larger sand dunes. When the dunes pile up, the dune slope angle reaches a critical point, and once this tipping point is reached, continuing to pile up sand causes the dunes to slide down in the form of an avalanche. At the highest point before slipping, the sand pile is in a critical state between order and instability.
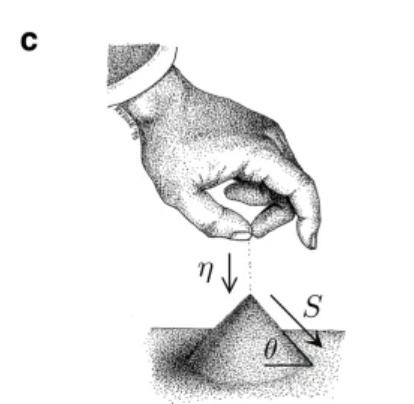
Figure 1. Schematic diagram of a sand pile in a critical state
In the sand pile model, by adding gravel to the sand pile at a rate η, the sand pile inclination angle θ becomes larger, and only a small amount of sand grains fall at first, but as the inclination angle θ approaches the critical angle θc, the number of sand grains S increases rapidly, until θ = θc, and the sand added will cause an avalanche collapse, causing the sand mound inclination angle θ to become smaller and return to the critical state, the following figure describes its feedback loop.
Figure 2. Feedback graph composed of order parameter(S) and control parameter (θ) in the sand pile model
Self-organizing criticality, proposed by Per Bak, Tang Chao, and Wiesenfeld in 1987, states that small interactions between elements of a system will naturally reach a critical point without external intervention. At this tipping point, a small event can produce a catastrophic response. Self-organizing critical performance is observed in complex systems such as financial markets and transportation systems.
Biochemical reactions in cells, neuronal activation in the brain, communication networks of computers, sealed communities, these systems are all in a critical state between chaos and instability. Living near critical points has the advantage of adaptability, such as being able to process information more efficiently to respond quickly to environmental changes. Thus, a system can stabilize itself near a critical point through what is known as self-organized criticality (SOC).
2. Self-organizing critical state at the single cell level
Can critical states, as the key to multicellular cognitive systems, appear in single cells? Existing studies indicate that at the cellular level, enzyme networks may be in a critical state to improve their ability to adapt to stimuli. The study constructed a simple dual-gene network that successfully brought living cells (bacteria) into an auto-tissue critical state by designing the interaction between gene expression and regulatory parameters within the cell, and identified the minimum conditions required to form an auto-tissue critical state. The same approach can be used to achieve related functions, such as improving the delivery of anti-colorectal tumor drugs.
The study's E. coli contained the GFP-lva gene, which is expressed as a protein σ, and the protein σ is degraded by the intracellular proteolytic mechanism (ClpXP), which affects the expression of the GFP-lva gene. Its regulation mechanism is shown in the following figure:
Figure 3. The regulatory mechanisms that synthetic cells have
When the gene expression rate is η (horizontal axis variable) is high, the bacteria produce too many proteins and are crowded (the light green part of the figure below), while if the gene expression rate is low, the bacteria are in a free state. By adjusting the gene expression rate η, the critical expression rate ηc that divides the critical state and the crowded state can be determined, when the bacteria are in the self-organizing critical state, where any small change will have a significant effect on the concentration of protein σ in the environment.
Figure 4. The critical position of E. coli is determined by the GFP-lva gene expression rate η change
In E. coli, the introduction of another protein that also competes for ClpXP will lead to a feedback loop similar to the sand pile model, resulting in a self-organizing critical state, which is a schematic diagram of the regulatory model of the dual gene composition in the study.
Figure 5. The negative feedback loop consisting of two genes (e), with σ1 as the order parameter and σ2 as the control parameter (f)
3. The significance of the self-organizing critical boundary for synthetic biology
The self-organizing critical phenomenon that occurs in nature hides the secret of the efficient and stable operation of organisms. The enzyme network presents a critical state when the substrate input rate is balanced with the processing capacity of the enzyme network, thus forming an adaptive advantage. However, the minimum conditions required to achieve a critical state remain unclear, and the study points to the smallest modulus in the gene network required to achieve a critical state.
Biocomputing refers to the information processing task through the biochemical reaction in living cells, the study design is in a critical state of cells, can amplify the signal amplitude in biological computing, rapidly form an avalanche signaling, thereby enhancing the development of biocomputing related applications. Since both multicellular cognition and swarm intelligence involve critical states, the study could support future research in relevant fields. At the same time, the study is also helpful for physical modeling of intracellular biochemical activity in three-dimensional space.