This article is from the WeChat public account: X-MOLNews
Chiral phenomena are ubiquitous in nature, ranging from galactic spiral arms, planetary rotations, atmospheric cyclones, to mineral crystals, organic molecules, and weakly interacting cosmona non-conservation. Interestingly, chirality also plays an important role in the process of human perception of different tastes. For example, the signature smells of passion fruit and wine may be related to the enantiomeric composition of their flavor molecules. As early as the 1970s, scientists reported on some enantiomers with odor differences, such as citronellol, linalool and carvacrol. Today, I would like to take you through a review of the relationship between flavor compounds and chirality by Professor Karl-Heinz Engel of the Technical University of Munich in Germany, which elaborates on five studies: analytical techniques, authenticity (anti-adulteration) assessment, chiral biosynthesis, structure and odor relationship, and odor receptors (Figure 1).
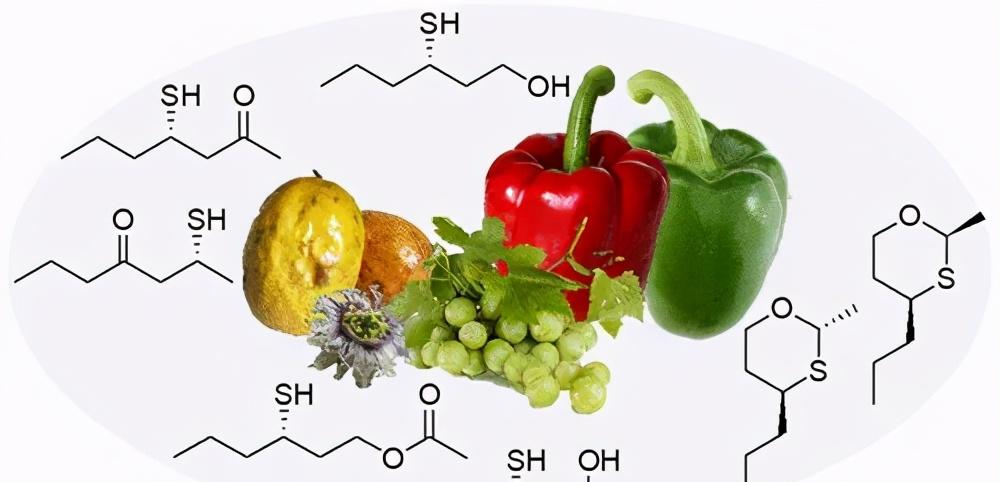
Figure 1. The connection between the quality of foods such as passion fruit, wine (juice), and bell peppers and the chirality of flavor molecules. Image credit: J. Agric. Food Chem.
In fact, it is still challenging to analyze and identify enantiomer mixtures of natural origin in flavor compounds. An ideal approach is to use diastereomer-derivatized capillary gas chromatography (GC) analysis. For example, this method is used to study the enantiomeric components in specific varieties of passion fruit. Both yellow and purple passion fruit contain free secondary alcohols, and the flavor components of the two passion fruit are separated by solid - liquid chromatography, one of which contains free alcohols and the other component contains 2-heptyl ester, the latter is saponified, the two components are derived with Mosher reagent, and then separated by GC chromatography, the results show that the free alcohols contained in yellow passion fruit are mainly (S)- enantiomers, while the 2-heptan present in purple passion fruit is mainly (R)- enantiomers.
At the same time, various derived cyclodextrins (CDs) as chiral stationary phase materials have become an important tool for GC to study food flavor molecules (Figure 2). Specifically, the primary hydroxyl group at 6 positions is silylated, and then derivatized on the 2 and 3 positions of the secondary hydroxyl group, so that it is possible to generate an ether or ester type stationary phase containing different substituents (FIG. 2A), wherein the most common derivatization strategy is to bind acetal, thereby generating alkoxymethyl derivatives (FIG. 2B), and these CD derivatives have been shown to be general chiral stationary phases.
Figure 2. Derivatized cyclodextrin structure as a GC chiral stationary phase material. Image credit: J. Agric. Food Chem.
In addition, chiral flavor compound enantiomeric components are potential indicators of food authenticity assessments and are promising to be used to identify food adulteration. For example, studies have found that methyl 2-methylbutyrate and ethyl 2-methylbutyrate in apples and strawberries are both biotransformed from L-isoleucine and therefore have a (S)-configuration. The 2-methyl branched-chain flavor compounds in fermented foods are also degraded by L-isoleucine. In apple fruit, (E)-2-methyl-2-butenyl ester is formed by biological hydrogenation reduction of (R)-2-methylbutyl derivative. Other studies have found that there is an "in vivo kinetics split" of 2-methylbutyric acid. The preferential formation of (R)- methyl ester results in an initial proportion of up to 35% of the enantiomer in methyl 2-methylbutyrate (R)- enantiomers. Conversely, the proportion of (S)-enantiomers in the remaining acid increases. That is, a decrease in (R)-acid eventually leads to an increase in the enantiomer of (S)-ester. This suggests that the absolute configuration of 2-methylbutyric acid and its methyl ester depends largely on the time point of bioreferencing and reflects the balance of various biosynthetic pathways. It also shows that chiral authenticity assessment must take into account the variables brought about by different biosynthetic pathways, developmental stages and production processes.
Sulfur-containing flavor compounds in food, especially polyfunctional thiols, play an important role due to their strong and unique odor and low odor threshold. One class of chiral sulfur-containing flavor compounds that has attracted great interest from researchers for decades is 3-mercaptohexanol and its derivatives (Figure 3). In a study of Sauvignon blanc white wines, researchers found that the enantiomer ratio of 3-mercaptohexanol and 3-mercaptohexyl ester may affect the sensory properties of the wine as well as consumer preferences. Another advance is the detection of non-volatile precursors of 3-mercaptohexanol and the identification of various (S)-glutathione conjugates as well as other intermediates involved in the biosynthetic pathway of 3-mercaptohexanol. These conjugates play a central role in the biosynthesis of sulfur-containing volatiles and are valuable odor precursors in the fragrance industry.
Figure 3. Sulfur-containing chiral flavor compounds identified from passion fruit. Image credit: J. Agric. Food Chem.
Scientists elucidated the biosynthetic pathway of the 3-mercaptohexanol and 3-mercaptohexyl ester enantiomer mixtures reported in Sauvignon blanc white wine and grape juice through stable isotope analysis and high performance liquid chromatography-mass spectrometry/mass spectrometry (HPLC-MS/MS). Specifically, glutathione (GSH) and (E)-2-hexenal are conjugated and reduced to form glutathione-3-mercaptohexanol (GSH-3-MH), which is further degraded by enzymes to form cysteine-3-mercaptohexanol (Cys-3-MH). It is then lyzed under the catalysis of β-lyase to generate 3-mercaptohexanol, followed by esterification of acetyltransferase to form 3-mercaptohexyl ester. The study found that the enantiomer ratio of glutathione-3-mercaptohexanol in Sauvignon blanc white wine and grape juice was 80% (S)/20% (R), and in subsequent biotransformation pathways, the (S)-enantiomers of Cys-3-MH always prevailed (Figure 4).
Figure 4. Stereochemical composition of 3-mercaptohexanol, hexyl 3-mercaptoacetate, glutathione and cysteine conjugates in Sauvignon blanc wine and grape juice. Image credit: J. Agric. Food Chem.
In addition to studying biotransformation pathways, polyfunctional thiols are also ideal for studying structure-odor relationships. Representative foods containing such compounds are bell pepper (Capsicum annuum) and cheddar cheese, and the results of the study show that: (1) the separation of stereoisomers of β-mercaptoal alkone and β-mercaptoacetan can be achieved with derivatized cyclodextrin material as the stationary phase; (2) kinetics splitting combined with enzyme catalyzed and NMR analysis of diastereomer derivatives can identify the absolute configuration of the compound; (3) the odor characteristics of stereoisomers can be determined by gas chromatography - smeller (GC/ o) to make an assessment. For example, the odor thresholds and odor properties of the individual stereoisomers of β-mercaptolkone and β-mercaptoalkol can be determined simultaneously using a GC/O chiral stationary phase. As shown in Figure 5, 4-mercapto-2-alkone and 4-mercapto-2-alkyl alcohol have a methyl substituent R4 and show a tropical fruit flavor; these olfactory sensory descriptions are absent in R4-free 2-mercapto homologues. In addition, R4 determines the influence of asymmetric central configuration on odor thresholds and odor properties.
Figure 5. Comparison of the organoleptic properties of β-mercaptolkone with β-mercaptolkalkol. Image credit: J. Agric. Food Chem.
Based on cell odor receptor analysis, the researchers constructed a new way to study structural odor relationships and olfactory mechanisms. Screening 391 human receptors through cell luminescence analysis found that OR2M3 is a highly specific and adjustable odor receptor based on which people can sensitively feel the flavor compound contained in onions (3-mercapto-2-methylpentane-1-ol).
Although Professor Karl-Heinz Engel mentioned that these research examples are not comprehensive and objective because of the personal preferences of the researchers. But there is no doubt that chiral compounds will occupy an important place in the future of food science flavor chemistry research.
Chirality: An Important Phenomenon Regarding Biosynthesis, Perception, and Authenticity of Flavor Compounds
Karl-Heinz Engel
J. Agric. Food Chem., 2020, 68, 10265–10274, DOI: 10.1021/acs.jafc.0c01512
(This article is contributed by Mizumura Yamaku)