Diabetics desperately need more personalized treatments and management devices. Recently, it has been announced that the first mobile phone control insulin pump has appeared.
The U.S. Food and Drug Administration (FDA) has approved the smartphone app capable of initiating insulin delivery on iOS and Android operating systems. It is reported that the application belongs to Tandem Diabetes Care (hereinafter referred to as Tandem) and can be associated with the company's previous t:slimX2 insulin pump.
Tandem Diabetes Care is a medical device manufacturer based in San Diego, California, USA. The company is committed to developing medical technologies for the treatment of diabetes, especially insulin infusion therapy.
In August 2017, Tandem officially launched the t:slim X2 insulin pump. Wearing the device, patients can intuitively know their blood glucose values at any time through the display and do not require additional data reception facilities. Compared to other insulin pumps, the advantages of the t:slim X2 are clear.
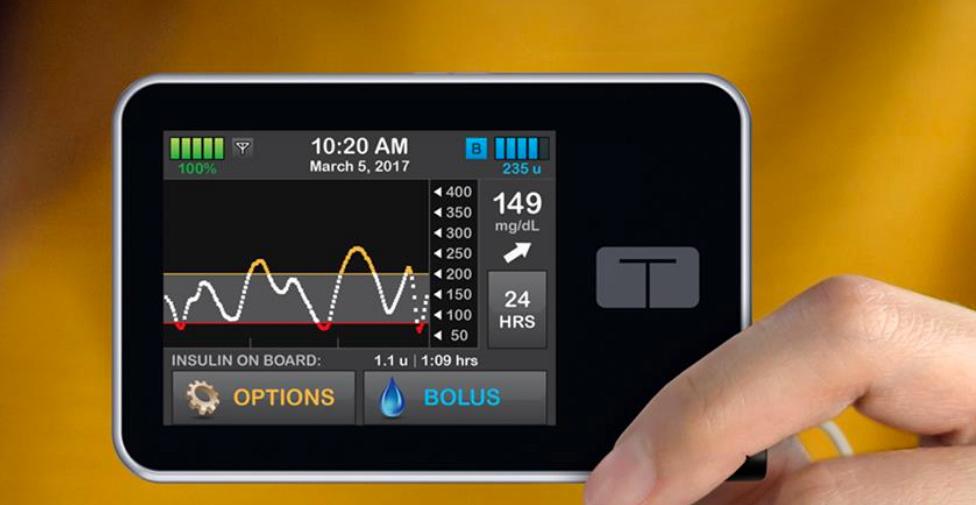
Figure | t:slim X2 insulin pump (Source: Tandem Diabetes Care official website)
In the time that followed, Tandem made several major upgrades and updates to give it more features.
For example, the t:slim X2 insulin pump uses Basal-IQ predictive low glucose suspension technology to predict and help prevent hypoglycemia; this insulin pump has a slim and stylish design and is the only pump compatible with the ambulatory blood glucose meter Dexcom G6 system, suitable for making treatment decisions without finger sting.
This time, Tandem's new move shows that the company has made new progress in the clinical treatment of insulin pumps.
It is understood that after FDA approval, t:slim X2 users will be able to "program or cancel insulin injections" using an app for iPhone and Android devices.
Previously, the app could be used to monitor trends and historical data, but not to initiate insulin delivery. Now, not only does it give patients a clear picture of blood glucose measurements and insulin delivery over the past 24 hours, but it also acts as an extension of the pump, providing mobile push notifications while the pump initiates an alarm.
John Sheridan, President and CEO of Tandem, said in an interview with the media: "The FDA approval passes one of the features we need most in need of enhancements, further validating our commitment to the innovation and diabetes community. ”
He also said in a statement that the most common scenario for current diabetics to interact with their pumps is to inject insulin during meals. With tandem's Control-IQ technology improving diabetes management, smartphone apps provide a more convenient solution.
Especially for patients who are reluctant to wear complex devices in public, the changes can be significant. Diabetes management is made easier and more discreet because the app reduces the number of direct interactions with pumps and allows users to monitor alerts and blood glucose levels via their phones.
Among them, Control-IQ mentioned by Sheridan is an automatic conveyor system that Tandem has been trying to update its algorithm since it obtained application license in 2016.
It's important to note that the app actually operates independently of the pump, which means that the user can still program the dose, check the alarm, and monitor the pump's own blood glucose levels.
(Source: Tandem Diabetes Care website)
In fact, Tandem isn't the only medical technology manufacturer that aims to make diabetes management more compatible with smart mobile devices.
In 2017, Amalgam Rx, a partner of biopharmaceutical company Novo Nordisk, launched iSage Rx, a mobile program for insulin determination, which helps people with type II diabetes calculate insulin doses.
However, iSage Rx is primarily aimed at patients who can self-manage insulin without relying on automatic pumps, which is the biggest difference between it and Tandem's new application, because it does not actually control the delivery of drugs precisely.
Ryan Sysko, founder and CEO of Amalgam Rx, said: "Although we developed an app to test the efficacy of iSage Rx, our ultimate goal is to provide insulin dose calculation services. We hope that companies that are creating diabetes surgical systems will be able to easily integrate the iSage Rx insulin dose calculation service. ”
In addition to that, the most noteworthy thing is that Tandem claims that it will make this feature available to existing users for free.
It is reported that the application function will be updated through the remote software of the t:slim X2 insulin pump, which in turn will connect to the smartphone. The specific release time may be divided into two batches, first limited to the U.S. this spring, and then extended to other eligible t:slim X2 users in the summer.
Currently, the feature is only compatible with the iPhone 12 running iOS 14 or the Samsung Galaxy S20 running Android 11, though Tandem says it's working to add more smartphone models and operating systems to the software.
In addition, Apple has also shown considerable ambitions in the field of diabetes treatment.
According to a report, the current Apple CEO Tim Cook was seen actively wearing a prototype glucose tracker around the Apple campus.
But it is clear that as things stand, Apple has encountered considerable obstacles in this area. In particular, the FDA approval coincided with Apple's exploration of the potential application of Apple Watch for diabetes treatment, which may cause greater pressure on Apple.
-End-
reference:
https://www.theverge.com/2022/2/17/22938844/fda-insulin-delivery-smartphone-app-tandem?scrolla=5eb6d68b7fedc32c19ef33b4https://en.wikipedia.org/wiki/Tandem_Diabetes_Care
https://9to5mac.com/2022/02/17/fda-clears-the-first-iphone-app-for-delivering-insulin-rollout-starts-this-spring/