Editor's note: As the first soluble diinsulin formulation, DeguMendon bisin was launched in China in 2019. Degu aspart biinsulin preparation can better simulate the "biphasic unipolar" of insulin physiological secretion, and the insulin component that is, insulin aspart can quickly take effect during meals and control postprandial hyperglycemia, while the basal insulin component, degu insulin, can better control fasting blood glucose, so it can take into account fasting and postprandial blood glucose control, and also shows unique advantages in reducing nocturnal hypoglycemia.
First, the unique mechanism of Degu asparticol
Degu Mendon double insulin is a soluble preparation, no turbidity, no need to mix when injecting, which is also its unique advantage over the previously listed premixed insulin analogues, which greatly improves the convenience and flexibility of insulin use in diabetic patients; in addition, the preparation contains 70% of the ultra-long-acting basal insulin analogue (Degu insulin) and 30% of the rapid-acting insulin analogue (aspart), the two components exist independently in the preparation, and can play a role alone after subcutaneous injection.
Second, Degu Mentong double insulin eliminates the "shoulder effect" and controls sugar more smoothly
Among the insulin preparations currently available, the proportion of premixed insulin is very high due to its low price, but it is difficult to avoid the "shoulder effect" in its use. So, what is the "shoulder effect"? Premixed insulin contains a fixed proportion of protamine-binding and non-protamine (soluble) insulin, after injection, insulin bound to protamine is slowly absorbed from the reservoir into the circulation, free insulin is rapidly absorbed to play the role of meal insulin, the two components are not completely independent, the rapid action of the hypoglycemic effect is prolonged, resulting in the effect of the medium-acting component of the premixed insulin and the effect of the meal component superimposed, which produces a "shoulder effect" and increases blood glucose fluctuations.
At present, a large number of clinical studies have not observed the "shoulder effect" of degu asparticil. This is mainly because the double hexamer formed by the action of zinc ions in the side chain is a highly stable structure. In the case of higher concentrations of zinc ions, whether in the preparation or after injection into the subcutaneous reservoir, there may be little or no interaction between degu insulin monomer and insulin aspart monomer. This soluble double insulin shows superior PK characteristics in initiation insulin therapy [1] over conventional premixed insulin[1], with degu insulin components enabling a smooth and long-lasting basal insulin supply while meal-time fast-acting insulin components can function independently.
3. Recommend degu asparticle as the choice of initiation insulin therapy for patients with T2DM
A Japanese real-world study[2] found that Degu aspart double insulin therapy was well tolerated, there were no severe hypoglycemic events, and the initial application was significantly associated with good glycemic control and high compliance rates in patients. A total of 1321 patients with diabetes were included in the study, of which type 2 diabetes mellitus (T2DM) accounted for 95.2%, 973 patients had HbA1c data and 319 patients had fasting blood glucose data at 1 year of medication. The results showed that HbA1c decreased by 0.51% from baseline after 1 year of degu asparticinization (the P1c reduction trend occurred as early as 3 months of treatment (Figure 1); among them, the decrease in HbA1c in patients with T2DM was more significant, with a decrease of 0.52% after 1 year of treatment (P
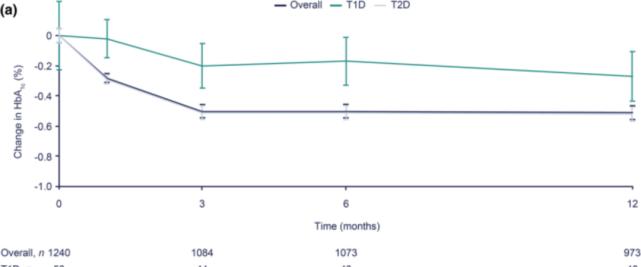
Figure 1. Degu mendon double insulin treatment with average HbA1c for 1 year
Figure 2. 1 year after initiation of de gumenpartein double insulin therapy, the decline in HbA1c was greater in patients with T2DM
The efficacy was also consistent at 1c, with a decrease of 0.57% and 0.56%, respectively; in patients over 75 years of age, the decrease in HbA1c was slightly smaller (-0.39%), but there was a statistical difference in the decrease in HbA1c in all age groups (P
Compared with baseline, there was a significant decrease in abdominal blood glucose at 6 months (n=415) and 1 year (n=319) on degumeneulin(s)(-28.1 mg/dl and -32.1 mg/dl, respectively, with PPP).
Another 12-week real-world study from Japan[3] included a total of 115 patients with T2DM to evaluate the efficacy and safety of T2DM patients from twice-daily premixed conventional insulin (group 1, n=55) or multiple injections of intensive insulin (group 2, n=60) to twice-daily degumenton double insulin therapy, for inter- and intra-group comparisons. The results showed that the glycemic control, median daily total insulin dose, body mass, body mass index and hypoglycemic events in groups 1 and 2 were significantly improved (P=0.001). There is no difference between other parameter groups (P>0.05). Therefore, this real-world study data analysis showed that degu asparticle improved prognosis in patients with T2DM and that significant benefits were observed in terms of total daily insulin requirement, body weight, and hypoglycemia.
Degu Mendon double insulin has a stable effect, the short-acting insulin and basal insulin components are clearly separated, and the problem of fasting and postprandial blood glucose can be solved with a single injection. Degu asparticin has been conducted in several clinical trials worldwide, and a large number of research data have defined its clinical efficacy in improving glycemic control in patients with T2DM, and its safety and tolerability are higher than that of traditional insulin.
Compared with traditional insulin, Degu Aspart double insulin has a better hypoglycemic effect and less hypoglycemia at night
A 26-week study of patients treated with conventional insulin in previous periods included premixed human insulin, premixed insulin analogues, self-blended insulin with 20% to 40% rapid-acting components, short-acting insulin, and conversion to degumenxon bisin insulin therapy. Results showed significant improvement in HbA1c in patients treated with degu aspart, particularly fasting glycemic control, and lower incidence of overall and nocturnal hypoglycemia [4]. If the patient has poor glycemic control (i.e., HbA1c>8.0%), the conversion to degumendon can be done in 1:1 dose units when switching to degumendon bisin insulin with a previously used insulin premix regimen. If the patient is being treated with a premixed insulin analogue, the equal dose conversion is split into degu asparticle double insulin twice daily, administered with the main meal; if HbA1c levels ≤ 8.0% or the patient has experienced hypoglycemia, the starting dose of degu aspartic bis insulin should be reduced by 10% to 20% compared with the dose of premixed insulin received before the regimen conversion.
Nocturnal hypoglycemia is a complication in insulin therapy, and it is also an obstacle to intensive insulin therapy in some patients, which will adversely affect the quality of life of diabetic patients and even bring serious clinical consequences. Compared with traditional long-acting insulin, degumeneulin significantly reduced the risk of nocturnal hypoglycemia, which is associated with a very stable PK/PD (pharmacokinetic/pharmacodynamic) spectrum of hypoglycemia, with lower blood glucose variability and less risk of nocturnal hypoglycemia during treatment. The Step by Step study[5] confirmed that degumenson double insulin is similar to traditional long-acting insulin with lowering hypoglycemic efficacy, with fewer injections, significant reductions in nocturnal hypoglycemia, and lower insulin doses. At weeks 26 and 38 of treatment, HbA1c met the standard (1c reduction (ETD = 0.07%, 95% CI: -0.06 to 0.21) and the overall incidence of hypoglycemia was similar, but there was a significant reduction in nocturnal hypoglycemic events in patients treated with de Gumenpart (ERR = 0.61, 95% CI: 0.40 to 0.93).
Basal insulin doses of 36 to 40 U (or 0.5 IU/kg/day) are recommended, after which conversion to degu asparticle or other regimens may be considered if glycemic control remains poor, HbA1c ≥ 7.0%, and postprandial blood glucose ≥ 180 mg/dl (≥10 mmol/L). An important issue to consider when switching from basal insulin to degu asparticle is that the dose unit is not necessarily a 1:1 conversion, and patients who have experienced hypoglycemia or have previously received conventional insulin therapy may need to reduce the dose. The Step by Step study provides clinical guidance on how, when, and why patients with T2DM are treated with degu asparticle to achieve their target HbA1c. Degu asparticin is titrated according to the "precise sliding ratio" algorithm. The Degu asparticle double insulin algorithm is similar to the basic insulin therapy algorithm, and studies have shown that degu asparticle double insulin is as effective as multiple insulin injection intensive therapy in intensive insulin therapy, with a more ideal advantage of reducing the risk of nocturnal hypoglycemia.
5. Conclusion
Degumenton double insulin provides both basal and mealtime insulin coverage. This di-insulin formulation is more flexible and has fewer injections than the basal-meal insulin regimen. The initiation regimen of Degumenton double insulin is simpler and more adaptable in diabetic patients, and can be given as a single injection or twice a day. Real-world studies have found that it is better suited to the lifestyle of people with diabetes compared to more complex insulin alternatives, and patients are very much comfortable with insulin therapy with lower daily injections and higher adherence. Clinical evidence supports the widespread use of degu asparticle in T2DM populations as an insulin initiation or intensive treatment option [6].
bibliography:
1.Kalra S, et al. Advances in therapy, 2018, 35(7): 928-936.
2.Katabami T, et al. Advances in therapy, 2021: 1-18.
3.Özçelikik S, et al. Archives of Medical Science: AMS, 2021, 17(1): 1.
4.Fulcher G R, et al. Diabetes Care, 2014, 37(8): 2084-2090.
5.Philis-Tsimikas A, et al. Diabetes research and clinical practice, 2019, 147: 157-165.
6.Mehta R, et al. Diabetes, Obesity and Metabolism, 2020, 22(11): 1961-1975.