Original White Feather Minnet
Great content
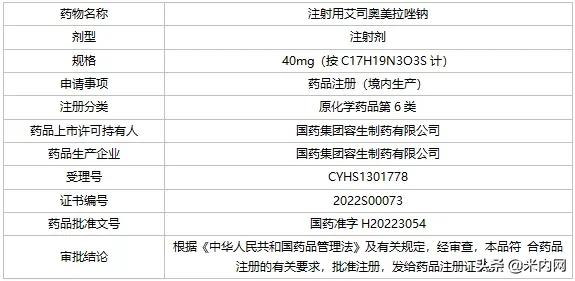
On February 18, Sinopharm Hyundai issued an announcement that its wholly-owned subsidiary, Sinopharm Group Rongsheng Pharmaceutical, received the registration certificate of esomeprazole sodium for injection approved and issued by the State Food and Drug Administration, and the project has invested a total of 3 million yuan in research and development costs. According to data from The Intranet, sales of esomeprazole sodium for terminal injection in public medical institutions in China exceeded 3 billion yuan in 2020, and the first half of 2021 increased by more than 25% year-on-year.
Basic information on medicines
Esomeprazole sodium for injection can be used as an alternative to gastroesophageal reflux disease when oral therapy is not available, reducing the risk of rebleeding after endoscopic treatment of gastric and duodenal ulcer bleeding in adults and preventing bleeding from stress ulcers in severe patients.
Sales of esomeprazole sodium for terminal injection in public medical institutions in China in recent years (unit: 100 million yuan)
Source: Intranet China's public medical institution terminal competition pattern
Esomeprazole sodium for injection was developed by AstraZeneca, which was first listed in Sweden in 2003 and approved in China in 2007. According to intranet data, in 2020, the sales of esomeprazole sodium for terminal injection in China's urban public hospitals, county-level public hospitals, urban community centers and township health centers (referred to as China's public medical institutions) exceeded 3 billion yuan, an increase of more than 25% year-on-year in the first half of 2021, and AstraZeneca had the largest market share, more than 40%.
Source: Intranet Consistency Evaluation Database
At present, more than 40 enterprises have the production approval of esomeprazole sodium for injection, of which 18 have been evaluated by Liaoning Haisco Pharmaceutical, Hainan Beite Pharmaceutical, Chia Tai Tianqing Pharmaceutical Group, Chongqing Laimei Pharmaceutical and so on. In addition, yichang Dongguang Yangtze River Pharmaceutical, Lepu Pharmaceutical and other 5 consistency evaluation supplementary applications in the review and approval, Shandong Lukang Pharmaceutical | Hangzhou Aoya Biologics, Shandong New Era Pharmaceutical, Hunan Hengsheng Pharmaceutical and other 7 in the imitation of 4 types of production in the review and approval, after approval is regarded as the same evaluation.
Sinopharm Hyundai said that the sinopharm group Rongsheng Pharmaceutical obtained the registration certificate of esomeprazole sodium for injection, which will further enrich the company's product line in the field of digestive system and help meet market demand. Up to now, the project has invested about 3 million yuan in research and development costs.
Source: Company announcement, Intranet database
Note: As of February 16, if there is any omission, welcome to correct!