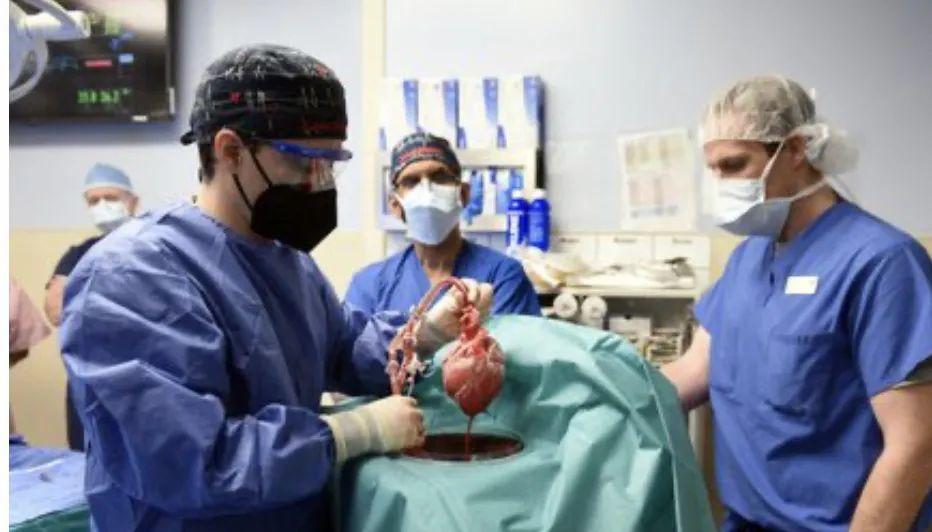
On January 7, a team at the University of Maryland School of Medicine reported the world's first transgenic porcine heart transplant for a male patient with advanced heart disease. It has been a week since the surgery began, and the industry is highly concerned about how the patients are doing.
The first financial reporter learned from Professor Wu Zhongjun, director of the Artificial Organ Laboratory at the University of Maryland, on January 15 that the patient had left ECMO (extracorporeal membrane oxygenation) two days ago and began to walk on the ground a day ago.
Xenotransplantation human trials are necessary
According to Muhammad Mohiuddin, a surgeon at the University of Maryland who leads the transplant research team, the team will monitor the patients' immune response and heart function and continue with controlled clinical trials. If a suitable patient shows up, they hope to be able to apply for more emergency procedures for xenotransplantation surgery.
Patients respond well one week after receiving transplants with no acute rejection, meaning that xenotransplantation of genetically modified organs is one step closer to success and will provide a wealth of data on the likelihood of xenotransplantation, although there are still many ethical and technical barriers to this cutting-edge technology.
To date, most xenograft studies have been conducted in non-human primates. The researchers hope that the first porcine-human xenotransplantation procedure will facilitate the launch of human clinical trials for xenotransplantation and help push it to address ethical and regulatory issues.
Academician Ge Junbo of the Department of Cardiology of Zhongshan Hospital affiliated to Fudan University commented: "This event is an important milestone in the history of human organ replacement therapy. Not only end-stage cardiovascular disease, but also epoch-making significance for other organ replacement therapies. ”
Last year, surgeons at NYU Langone Health transplanted kidneys from the same genetically modified pig into two cases of brain-dead kidney disease, which were not rejected.
In this regard, Ge Junbo believes that because the complexity of the heart's tissues far exceeds that of the kidneys, the successful implementation of the first heart xenotransplantation operation is of greater significance.
"From a few human patients, we learned things that we couldn't learn from dozens of monkeys." David Cooper, a transplant surgeon at Massachusetts General Hospital in Boston, said, "It's time to walk into the hospital and see how these hearts and kidneys are performing in patients." ”
The researchers say it's important to study xenotransplantation in humans rather than animals because animal models are restrictive. Jeremy Chapman, a retired transplant surgeon at the University of Sydney in Australia, said: "Differences between species prevent us from using the model further to predict clinical outcomes, due to the fact that non-human primates tend to have antibodies that humans do not have that attack proteins on pig organs. ”
In addition, researchers need to be able to study the physiology of a pig's heart, such as whether it beats at the same speed as a human heart.
Gene editing testing is expensive
In recent years, with the advent of gene-editing technology CRISPR–Cas9, xenotransplantation has made significant progress. The latest transplant at the University of Maryland Medical Center (UMMC) used ten genetically modified pig organs. Although the combination seems to have worked, it is unclear how much genetic modification is necessary.
Professor Megan Sykes, a surgeon and immunologist at Columbia University in New York, said: "Evaluating every genetic modification requires more validation of scientific data. We need this information because some gene editing can also be harmful to the human body. ”
In addition, xenotransplantation is still limited by the supply of genetically modified pigs as well as regulatory hurdles. The U.S. FDA needs to ensure that these GM pigs meet very strict standards for medical-grade facilities. The only company in the U.S. that can supply such GM pigs, Revivicor in Blacksburg, Virginia, can supply GM pigs that meet the standards of medical-grade facilities and clinical use.
Revivicor CEO David Ayares revealed that the company's pigs are currently being raised at a facility near Birmingham, Alabama, and the company is preparing to build a larger facility in Virginia, hoping to eventually supply hundreds of organs each year.
For two decades, Ayares has been working on the design of this genetically modified pig by testing various genetic modifications to assess how they limit rejection of xenotransplantation in humans and other primates.
But this genetic test is very expensive. It was revealed that transplanting pig hearts into baboons cost about $500,000 per transplant. Cooper of Massachusetts General Hospital believes that a viable model for future xenotransplantation could be genetically modified for specific organs and recipients.
Several other companies are also designing transgenic pigs for solid organ transplantation with different genetic modifications, such as eGenesis in Cambridge, Massachusetts, and NZeno in Auckland, New Zealand. Although these companies do not yet have medical-grade facilities, as more companies get involved, the cost of genetic testing trials is expected to fall in the future.