In recent years, many studies have shown that non-coding RNA that does not translate into proteins is also indispensable in a variety of cellular activities. Therefore, understanding the function and metabolism of RNA becomes crucial, and only when we have a clear understanding of the physiological function of RNA can it be possible to regulate and correct it in abnormal disease states.
However, RNA has the characteristics of dynamic and changeable, and its distribution is also specific in time and space, which also puts forward higher requirements for the study of RNA, and it is necessary to find a technology that can precisely regulate RNA to understand its function in cells.
Recently, Professor Yang Yi's team at East China University of Science and Technology has developed a light-controlled RNA-binding protein, which will bind to a specific sequence on the RNA, just like a photosensitive switch, and through the regulation of light, changes in RNA function and metabolism can be realized. The results were published in Nature Biotechnology. Chen Xianjun, the first author of this article, told Guo Hu: "Our team has been focusing on the development and application of optogenetic technology, and in addition to continuing to deepen cultivation in this field in the future, we also hope to promote the industrialization of technology as soon as possible." ”
How to achieve the binding of photomodulated proteins to RNA?
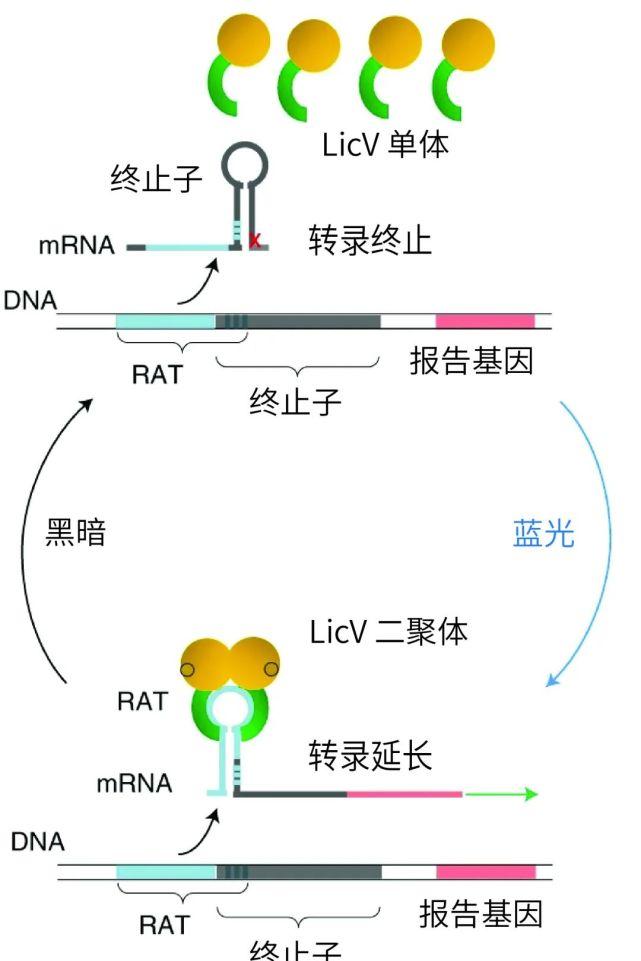
LicV monomers form dimers under blue light exposure and bind to RNA | Nature biotechnology[1], Chinese author of fruit shell
This new light-controlled RNA-binding protein consists of two core modules: the RNA-binding domain LicTCAT, a transcriptional anti-terminating protein found in Bacillus subtilis, which can specifically bind to ribonuclear anti-terminating RNA (RAT) sequences when the two LiCTTTs are dimerized, thereby preventing the formation of RNA-terminated stem-ring structures; and the other is vivid protein (VVD), which can rapidly dimerize under the activation of blue light.
These two core modules impart LittCAT-VVD fusion proteins to dimerize under the induction of light, thereby enhancing their ability to bind to RNA, and removing the light source will lead to protein dimers and gradual dissociation of RNA and proteins. Using red fluorescent protein as the reporter gene, the researchers tested the fusion protein containing different conjugates between LittCAT and VVD, found the fusion protein with the largest fluorescence intensity change in blue light irradiation and darkness, and named it LicV.
How does it work in different application scenarios?
Fusion proteins obtained by binding different effector proteins to LicV can affect the metabolism of RNA under light control| natural biotechnology[1], Chinese-chinese author of Fruit Shell
Since blue light can regulate the binding of LicV proteins to RNA sequences containing RAT, can this system have an impact on RNA function and metabolism? The researchers fused a series of effector protein domains with different functions with LizV to test whether blue light could make these effector proteins work accordingly.
Intracellular localization is the basis for RNA to function normally. The researchers tried to fuse LicV with a variety of different subcellular localization signals, and RNA containing THE RAT sequence and its reporter protein during blue light irradiation can appear in a relatively short period of time consistent with the localization signal. When darkness returns, this specific change in position will gradually disappear.
The splicing process of removing introns and merging exons after DNA transcription is an important step in the formation of coding RNA. Fusing LicV with splicing proteins that promote or hinder exon retention separately forms a light-controlled splicing factor, which, after lighting, can make the splicing reporter RNA appear different splicing methods.
RNA translation is the basic way organisms produce proteins. Fused with LicV with the translation initiation factor 4E, the translation of the reporting protein is significantly enhanced under blue light exposure, and the intensity of translation is also affected by the light intensity.
Degradation occurs after RNA completes function. The researchers fused LicV with barnase, a small RNA clipping module, and bound to the RAT stem-ring of the target RNA under blue light, giving it strong shear activity, but in the dark, the non-specific shear activity was negligible.
Through experimentation with a variety of scenarios, the team found that LicV is a wide range of light-controlled RNA-binding proteins that can be used to regulate RNA in time and space by fusing it with different effector proteins. Chen Xianjun told the fruit shell that because the effect factor effect is positively correlated with light intensity within a certain range, and VVD is most sensitive to light in the blue light region, by adjusting the light intensity and wavelength, it can even achieve quantitative adjustment of RNA.
A powerful combination
CRISPR technology is growing rapidly in the life sciences | Pictures HHMI.org
In recent years, the development of CRISPR/Cas9 systems has brought disruptive changes to life science research, and it is widely used in genome editing, high-throughput genome function screening, live-cell imaging and other fields. The Cas9 protein is guided by the guide RNA to precisely locate the target location within the genome.
So can Lilv be used in conjunction with CRISPR?
The researchers skillfully bound the RAT sequence to the guide RNA, and then combined the LicV protein with the transcriptional activation domain VPR to develop the LA-CRISPR joint system. Under the illumination of blue light, the expression of the target gene positioned by the guide RNA will be significantly increased, which not only plays the advantage of accurate localization of the CRISPR system, but also can achieve the effect of light control. The researchers also combined green fluorescent protein with LicV to achieve fluorescent labeling of genomic sites under light control through LA-CRISPR.
CRISPR technology has a certain degree of off-target effect, that is, some non-target locations in the genome become the target of CRISPR. So, are there the same technical problems with la-CRISPR systems? The hull learned from Chen Xianjun that researchers are conducting relevant research to reduce the off-target effect of CRISPR and achieve precise regulation of RNA.
Application prospects
RNA-based treatments could open up new opportunities to defeat disease | Science.org
The development of this light-controlled RNA-binding protein LicV has opened a new door to the study of the function of RNA to a certain extent. In current studies, the regulation of RNA-induced expression is often carried out by drugs, which are not only potentially toxic to cells, but also can only obtain information about a single regulatory mode of RNA expression.
Chen Xianjun also told The Fruit Shell: "LicV is based on the design concept of synthetic biology, from Bacillus subtilis and Neurospora crassa, which are biologically orthogonal to mammalian cell components and therefore have good biosecurity." At the same time, LicV binds to different effector molecules to make precise changes to multiple processes such as RNA localization, splicing, translation, and degradation.
In addition, the development of the LicV system is an opening of the research and development model, this protein is regulated by blue light, and perhaps in the future researchers can find receptor proteins that are sensitive to other spectral ranges. Imagine if different colors of light-controlled proteins are transferred into cells, the goal of regulating multiple RNAs through different light can be achieved, providing an effective tool for studying a variety of complex life activities, expanding the toolbox of life science research, and enabling scientists engaged in such research to more fully and comprehensively understand the function of RNA.
"RNA is the cutting-edge focus of current biomedical research, in addition to participating in almost all important life processes of cells, RNA itself can also participate in the prevention and treatment of diseases as a drug, such as mRNA vaccines, interfering RNA, etc." Therefore, the development of methods that can precisely control these RNAs will greatly promote the functional interpretation of RNAs and the development of RNA-based biomedicine. Chen Xianjun explained, "LicV-based RNA optogenetics technology can allow people to accurately control the transcription, splicing, localization, translation, degradation and other metabolic behaviors of RNA, compared with traditional methods, the method has excellent controllability, its time resolution can reach milliseconds, and the spatial resolution can reach subcellular size, which makes it avoid excessive treatment and insufficient treatment, and is expected to truly achieve precision medicine." ”
Currently, researchers are conducting experiments on the application of LicV to living tissue and have obtained preliminary results. Although this technology is still some distance away from clinical application, it is worth looking forward to in the future.
Thanks
Thanks to Dr. Chen Xianjun of the Yang Yi Research Group of East China University of Science and Technology for his review and suggestions on this article.
bibliography
Author: Guo Yixuan
Edit: Crispy fish
Typography: Yin Ningliu
Research team
The interdisciplinary research team of optogenetics and synthetic biology | Courtesy of Chen Xianjun
Corresponding author Yang Yi: Ph.D. graduated from Tsinghua University in biochemistry, and went to Harvard Medical School in the United States to conduct postdoctoral research on metallothioprotein after graduation. He is currently a Distinguished Professor of the School of Pharmacy and the National Key Laboratory of Bioreactor Engineering of East China University of Science and Technology, engaged in the development and application of cutting-edge technologies in synthetic biology and optogenetics.
Corresponding author Chen Xianjun: Ph.D. graduated from the School of Pharmacy, East China University of Science and Technology, majoring in chemical biotechnology and engineering. In 2015, he began to engage in postdoctoral research in pharmacy of East China University of Science and Technology. He is a fixed member of the State Key Laboratory of Bioreactor Engineering of East China University of Science and Technology, whose research direction is the development and application of new optogenetic technologies and the development and application of fluorescent labeling technology for biological macromolecules.