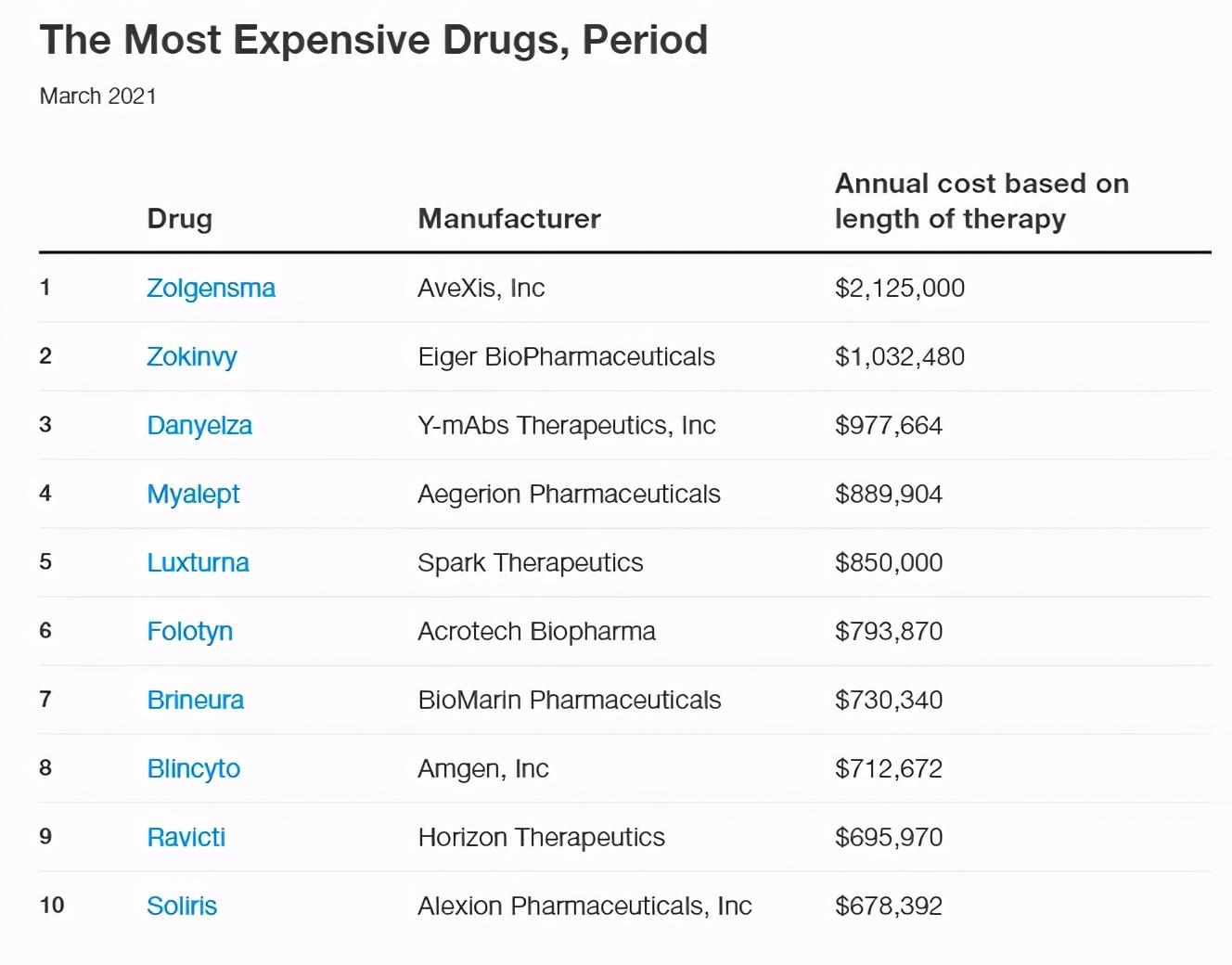
Every year, GoodRox, a U.S. drug price tracking site, dynamically updates its list of the most expensive drugs. Recently, GoodRx once again listed the 10 most expensive drugs on the market, including those sold in pharmacies as well as those used only in hospitals. The drugs on the list are price-based and may differ from the prices consumers pay at pharmacies or through drug support programs.
NO.1 Zolgensma
Novartis' gene therapy Zolgensma, which treats spinal muscular atrophy, topped the list with $2.125 million. At first glance, the cost of treatment may seem prohibitive. However, the current ten-year cost of SMA treatment is more than $4 million, for example, another Biogen-approved SMA drug, Spinraza, which cost $750,000 in the first year of treatment, and thereafter, the annual price for patients is $375,000, and will also reach $4.12 million in ten years.
The high price of drugs is more due to technical difficulties or the lack of effective drugs. Spinal muscular atrophy is one of the most common rare diseases, with about 11,000 infants presenting in 1. SMA currently has only 3 approved drugs, Zolgensma is a genetic drug, expensive and inevitable. Evrysdi, a small molecule drug approved in 2020, has an annual treatment cost of more than $300,000 at the highest recommended dose (5 mg, 2 years and older or more than 20 kg, qd).
Paying for Zolgensma isn't easy, and insurance companies may not be able to afford it; alternatively, insurance companies may have certain requirements to make patients eligible for coverage. To encourage insurers to underwrite Zolgensma, manufacturer AveXis allows certain insurers to pay in installments of $425,000 per year over a 5-year period.
NO.2 Zokinvy
Zokinvy, which was just approved for marketing in November 2020, is the first treatment developed by Eiger Biopharma for Hutchinson-Gilford Progeria syndrome (HGPS or Progeria) and Proger-like nucleostribinosis. Both indications are ultra-rare, inherited diseases of premature aging that accelerate mortality in younger patients.
Although the dosage depends on the surface area of the body, patients usually take about 200 mg of Zogenvy per day. At a price of 50mg capsules for $ 717, the monthly fee will reach $ 86,040. As a result, Zogenvy, like most orphan drugs, costs as much as $1032480 a year. But Eiger has implemented the ONECARE program to improve patients' ability to pay, which can provide insurance and financial support for patients.
NO.3 Danyelza
Danyelza is a new member of the Expensive Drug Club. The drug, also just approved in November 2020 by Y-mAbs Pharmaceuticals in New York, is used to treat pediatric patients with bone or bone marrow recurrence or refractory high-risk neuroblastoma at an annual cost of $977664.
Danyelza has accelerated the approval of the market, while the same target and indications of dextuximab has been fully approved, it can be said that there is a direct competitive relationship, dextuximab sales exceeded 100 million US dollars in 2020. Danyelza costs $20,368 per bottle, and patients typically use about 48 bottles a year, close to $980,000, so Y-mAbs also offers Y-mAbsConnect, an assistance program for some uninsured patients.
NO.4 Myalept
Myalept is used to treat systemic lipodystrophy and leptin deficiency with abnormal systemic fat distribution, and is the only drug on the market in the same target, and even the disease has no other drugs in the clinical stage, and patients have very few treatment options.
The price of Myalept increased from $71,306 per month to $74,159 in February this year, costing nearly $890,000 a year. Because Myalept is the only treatment available to control this rare disease, there is no other cost-saving alternative, but Aegerion Pharmaceuticals is also helping some patients with the cost of drug treatment.
NO.5 Luxturna
Luxturna is priced at $850,000 a year, ranking fifth. Luxturna is a gene therapy that treats hereditary retinal dystrophy, a disease that can lead to vision loss or even complete blindness. Luxturna is also the first AAV gene therapy approved in the United States, using AAV2 to deliver normal RPE65 genes into retinal pigment epithelium (RPE) cells to compensate for RPE65 gene mutations. Luxturna can only be used in hospitals and requires doctors to inject medication into each eye, but the list price is expensive, at $425,000 per bottle.
It is gratifying that the competition for hereditary retinal malnutrition is accelerating, and there are also a number of drugs under study that are in the late clinical stage or close to the later stage, and there are more than 20 clinical phase I drugs, which is expected to greatly improve the accessibility of patients.
NO.6 Folotyn
Folotyn is a dihydrofolate reductase (DHFR) inhibitor approved for the treatment of peripheral T-cell lymphoma at an annual cost of nearly $800,000. Folotyn was first listed in the U.S. in 2009 and approved in China in 2020.
DHFR is a key enzyme in the thymus deoxynucleoside (dTMP), an essential precursor to the synthesis of DNA, which plays an important role in cell proliferation and is an important target for tumor therapy. However, the competition for peripheral T-cell lymphoma indications is accelerating, with HDAC and CD30 drugs joining, coupled with the gradual expiration date of Focalyn's patent, which may accelerate Folotyn's withdrawal from the top list of most expensive drugs.
NO.7 Brineura
Brineura, a First-in-class enzyme replacement therapy drug, first marketed in 2017 for the treatment of advanced infant neuronal neurolipid fusion protein type 2 (CLN2) Batten disease (CLN2-Batten). Batten disease is a generic term for a rare and fatal genetic disorder of the nervous system, also known as neuronalceroid lipofuscinoses (NCLs), which reduces the ability of cells to clear metabolite molecules due to defects in specific genes. Depending on the gene in which the mutation occurs, Batten's disease can be divided into 13 species, called CLN1-8, 10-14.
CLN2 disease is a genetic disorder that primarily affects the nervous system. The signs and symptoms of this condition usually begin between the ages of 2 and 4 years. Initial features usually include recurrent seizures (epilepsy) and difficulty coordinating movements (ataxia), and muscle twitches (myoclonus) and decreased vision in affected children. CLN2 disease affects motor skills such as sitting and walking, as well as speech development. This condition can also lead to loss of previously acquired skills (developmental regression), gradual deterioration of intellectual disability, and behavioral problems. People with this condition usually need to use a wheelchair in their late childhood and generally do not live longer than a teenager. Brineura is used to treat a slow decline in walking ability in pediatric patients aged 3 years and older.
Due to technical issues, both for target and disease considerations, Brineura's competition is woefully under-competitive. In January 2021, the 300mg specification Brineura was again raised by 2.14% to $28,090, and the recommended dose was 300mg per 2 weeks, which means that the cost totaled $730,000 over the course of a year.
NO.8 Blincyto
Blincyto is a CD3/CD19 bispecific antibody developed by Amgen, marketed in the United States in 2014 for the treatment of relapsed or refractory CD19-positive B-cell precursor acute lymphoblastic leukemia. Blincyto employs cycles of administration, the first of which is called the induction phase, designed to reduce the number of cancer cells; the second to fourth cycles, called the consolidation phase, contribute to the growth of new healthy cells. At each stage, patients need to use different amounts of the drug, but typically about 168 bottles are required each year, at current pricing, reaching $4242 per bottle, for an annual cost of $712672.
Blincyto's sales in 2020 were $379 million, a decrease from 2019. Innovative therapies continue to emerge, the increasing number of therapeutic options available in the field of acute lymphoblastic leukemia, coupled with small molecule drug competition, and Blincyto's backline label, its market performance in 2021 is still uncertain, and in January this year, Blincyto also raised prices by 5.89%, which is to provide some driving force for subsequent growth.
NO.9 Ravicti
Ravicti (glycerin phenylbutyrate) is an upgraded therapy developed by Horizon for its same indication drug sodium phenylbutyrate, which patients need to take 132 bottles per year. In January 2021, Ravicti's list price rose by 4.8% to $5,273 per bottle, at an annual cost of $690,000 at the list price.
Disorders of the urea cycle are genetic conditions that cause high levels of ammonia in the blood. If left untreated, it can lead to confusion, coma, and even death. Phenylbutyric acid is mediated by the active metabolite phenylacetic acid (PAA), which forms phenylacetyl glutamine (PAGN) with glutamine in vivo, which, similar to urea, also contains two nitrogens that can be excreted with urine.
As an early product of Horizon, sodium phenylbutyrate is recommended at recommended daily doses, sodium salt intake exceeds guideline recommendations, Ravicti (phenylbutyric glycerin) not only avoids this problem, but also improves fat solubility. In addition, phenylbutyrylycerin is a pale yellow, odorless, tasteless oily liquid, while sodium phenylbutyrate has problems such as poor senses (salty, smelly), high sodium content, and large tablet volume.
NO.10 Soliris
Soliris costs $678392 per year for paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, myasthenia gravis, and optic neuromyelitis. Soliris's dosing regimen may vary depending on the patient's age and the disease being treated, but most patients require a maintenance dose of 1200 mg every 2 weeks; however, Soliris is at high risk of infection, with many patients having breakthrough haemolysis. As a result, Alexion (AstraZeneca) has developed a long-acting complement C5 inhibitor, Ultomiris (ravulizumab).
Ultomiris (ravulizumab), the first and only long-acting C5 inhibitor, injected every 8 weeks, has been approved for the treatment of paroxysmal sleepy hemoglobinuria and hemolytic uremic syndrome in adults.
Soliris achieved $4 billion in revenue in 2020, but growth slowed (+3%), while the improved Ultomiris expanded rapidly, breaking through $1 billion with more than 200% growth to become the new blockbuster drug. It also contributed to AstraZeneca's acquisition of Alexion for $39 billion at the end of 2020.
summary
In this list of the world's most expensive drugs TOP10, in addition to some familiar drug figures, such as 丫lept, Folotyn, Soliris, there are also some new members, including Zogenvy and Danyelza, who just went on the market at the end of 2020. The change in the world's top list of the most expensive drugs shows scientific and technological progress to some extent, reflecting the huge development cost of drugs that need to be recovered by higher market pricing, and also reflecting some social problems of high-priced drugs due to insufficient competition. It is gratifying that the vast majority of pharmaceutical companies are carrying out corresponding drug rescue and subsidy measures to meet the needs of patients as much as possible. It is hoped that advances in technology and the emergence of innovative therapies will continue to meet the needs and accessibility of patients.