Sven | Writing
Crab leaf | edit
"Is this [recruiting patients for clinical trials]?"
Not long ago, Wang Yanan, general manager of Houpu Pharmaceutical, went to a hospital in a fourth-tier city to talk about clinical trial cooperation, and as soon as he opened his mouth, the neurology director of the hospital asked a question that made him cry and laugh.
It's not an uncommon scenario in his work — even though his company has helped more than 30,000 patients get treatment opportunities through clinical trials of new drugs.
On November 10, 2021, the "Annual Report on the Status of Registered Clinical Trials of New Drugs in China (2020)" released by the Drug Review Center of the State Food and Drug Administration shows that a total of 2602 clinical trials were registered in 2020, an overall increase of 9.1% over 2019 (2386 cases).
Just yesterday, Chinese innovative pharmaceutical company BeiGene landed on the Science and Technology Innovation Board of the Shanghai Stock Exchange, becoming the world's first company to successfully list on the NASDAQ, the Hong Kong Stock Exchange and the Shanghai Stock Exchange.
These are two sides of the coin that exist simultaneously in the development of innovative drugs in China – the rapid development of the industry, and the clear and difficult to overcome the cognitive gap.
01
Finding subjects is getting harder and harder
Every other Wednesday, Xiao Wu, a doctor at the clinical trial center of the top three hospitals, will take a small half-day to screen patients in the hospital information system.
This seemingly simple job sometimes takes years to finally complete — in the previous period, putting up posters in the hospital, li roll-up treasure, official micro-push, finding recruitment companies to cooperate, screening patients from the medical record system are all ordinary and important daily tasks of Xiao Wu, but "even so, it is good to be able to enroll one or two patients a month," he told Cyberlan.
"Difficult to find people" is a common dilemma faced by clinical trial executives, and Wang Yanan has a deeper feeling about it.
"Whenever I talk to patients about clinical trials, I always see their faces full of resistance with the words 'I don't want to be a guinea pig.'" Wang Yanan spoke to Cyberlan.
The lack of understanding and incomprehension of new drug development and clinical trials exists more widely than imagined.
The director of the department of the grass-roots hospital once suggested that a patient who met the enrollment conditions should participate in the clinical trial that Wang Yanan was responsible for, and when the director of the department communicated with the patient, the patient asked the doctor in fear: "Why don't you treat me?" Let me go to Beijing, is my illness serious again? ”
The problems encountered by clinicians and Wang Yanan have been concretely presented under the big data.
The "Annual Report on the Status of Clinical Trials of New Drugs in China (2020)" discloses data: in the clinical trials registered in 2020, less than half (45.4%) of the participants were recruited within one year after approval; the clinical trials completed in 2020 were still dominated by Phase I clinical trials, with an average completion time of 95.7 days; and only 5 Phase III clinical trials were completed, with an average completion time of 176.6 days.
At the same time, the average number of registered drug clinical trial target enrollments (international multi-center clinical trials in terms of domestic target enrollments) was 320, of which 53.2% (783) of the targeted enrollment of clinical trials ≤100.
Drug clinical trials refer to drug studies carried out in the human body for the purpose of drug marketing registration to determine the safety and efficacy of drugs, usually including phase I,II., III., IV. clinical trials and bioequivalence trials; often from the beginning of Phase II clinical trials, it is necessary to screen eligible patients as subjects to enroll.
"Phase I., II., III. clinical trials are all premarket trials, and Phase IV clinical trials are post-marketing re-evaluation. Generally speaking, Phase I is mainly for the tolerance and pharmacokinetics of healthy subjects, and phases II and III are mainly for patients to do clinical trials. A researcher at a university drug research institute explained to Cyberlan.
"When conducting clinical research and development, recruitment is mainly the responsibility of the hospital participating in the clinical trial, we help clinical investigators understand the trial protocol and enrollment criteria, and the investigator evaluates and makes the decision whether to enroll." Some pharmaceutical company R & D personnel said.
Many interviewees believe that in recent years, the regulatory authorities have become more stringent in the design of clinical trial protocols, the requirements for subject patients have become more and more precise, and they are "aligned" with international clinical trial standards and ethics, which objectively makes the recruitment of subjects must meet greater challenges.
The gap problem of clinical trial institutions is more realistic - subject to the uneven distribution of medical resources, most of the domestic centers with clinical trial qualifications are concentrated in large hospitals in first-tier cities such as Beijing, Shanghai and Guangzhou, and the gap in clinical trial institutions is large, "more difficult and more difficult".
Some clinical trial research experts told Cyberland that there are more than 100 rheumatic immunology departments with clinical trial qualifications in China, and the concentration of rheumatic immune drug research and development enterprises of some monoclonal antibodies and small molecule inhibitors is more obvious, and there are multiple indications for clinical research on the same drug. For example, the drugs are clinically studied in ra (reactive arthritis), AS (ankylosing spondylitis), PsA (psoriatic arthritis) and other indications, and these indications are concentrated in the rheumatology and immunology department - which not only leads to the concentration of the number of research drugs, but also the high number of trials, which makes the already small number of certified departments worse.
On the other hand, the acceleration of pharmaceutical companies in the research and development of innovative drugs has continued, and popular varieties have even begun to fight each other.
CED data show that in the 2602 clinical trials registered in 2020, the homogenization of targets is more obvious, mainly distributed in PD-1, VEGFR, PD-L1, etc., and cell therapy is still dominated by CD19 targets.
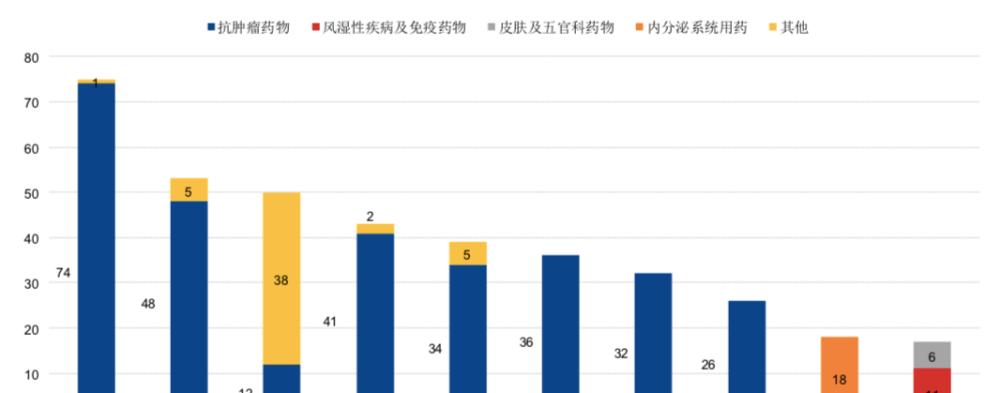
Top 10 targets and indications of drug varieties registered for clinical trials from 2016 to 2020 (data source: CDE, Dongxing Securities Research Institute)
"For various reasons, large research hospitals are unusually crowded, and the speed of clinical trials is unimaginable." Wang Yanan further analyzed to Cyberlan, "A hospital may undertake two, three or even more projects, and the specific implementation after the launch should be carried out in order, which will lead to a company or even several companies having too long clinical trials, and three years or even more to complete the recruitment of patients, seriously occupying the research and development lanes of other drugs." ”
02
The wind is coming, and so is the money
Fresh air outlet, blown from 20 years ago.
On the evening of November 10, 2001, China officially became a new member of the WTO as the then Chairman of the Organizing Committee of the Fourth World Trade Organization (WTO) Ministerial Conference, Yusuf Hussein Kamal, fell from the hand of the mallet.
Since then, the development of China's pharmaceutical industry has more possibilities.
After China's accession to the WTO and the United States experienced the bursting of the human genome bubble, a group of scientists represented by Wang Yinxiang, Zhang Xiaodong and Ding Lieming, founders of Beida Pharmaceutical, chose to return to China to start a business and devote themselves to the new drug research and development industry.
In 2015, the "traffic star" PD-1 broke into the public eye, and the first domestic PD-1 drug, Junshi Bio's triplemumab, was approved for clinical trials. Domestic companies such as Innovent Biologics, Hengrui Pharmaceutical and BeiGene followed suit, jointly ushering in the PD-1 era in China.
At the same time, a "license in" road that has been widely imitated for a long time to come is about to be vividly interpreted.
In 2016, Zaiding Pharmaceutical obtained a Parp inhibitor molecule (ovarian cancer drug) development right in China from abroad, and quickly listed it under the outlet of accelerated reform of drug approval and capital boost, thus exploring a commercial path to quickly introduce early pipelines in the license in mode and make quick money by taking advantage of the "poor efficiency" of drug research and development at home and abroad.
In 2018, the two major exchange science and technology innovation boards tore open their mouths for unprofitable biopharmaceutical companies, innovative pharmaceutical companies brought faster value-added realization imagination space, China's innovative drug industry was instantly pushed to the peak, and the associated clinical trial sites also felt the tide.
Domestic Drug Applications from 2016 to 2020 (Data Source: CDE, Dongxing Securities Research Institute)
In 2021, it became a typical PD-1, and at that time, it began to stir, and under the approval of a large number of declarations, tumor patients in major clinical research centers (hospitals) began to "outstrip supply".
"China's PD-1 is not for injection, it can be used for bathing." Some people in the industry issued such feelings about Cyberblue. CED data can support the fact that 75 drug varieties registered for clinical trials from 2016 to 2020 focused PD-1 targets.
The strong promotion of the payment end, the strong intervention of China's medical insurance, the world's largest payer, and the economic channels of medical insurance access negotiations have turned the commercialization and realization of a large number of innovative drugs into a reality that was once unimaginable.
The scene of the national talk (source: CCTV screenshot)
In the first quarter of 2020, even under the impact of the epidemic, the 89 medical insurance negotiation varieties in the implementation period still maintained a rapid growth momentum, with sales amount increasing by 23.30% year-on-year and sales volume increasing by 122.44% year-on-year, ushering in a good opportunity for market development through price for volume.
According to data from the intranet, most of the domestic 1 class 1 new drugs that successfully entered the medical insurance directory for the first time in 2019 have increased significantly in sales at the terminals of public medical institutions in China in 2020, such as Hengrui's pyrrolidinib maleate tablets, Xindec's xindilimab injection, which has become more than 1 billion varieties, and the first international first original research drug roxalistat capsules, which is incubated by China's local research and development, has soared by 6278.07%.
Although the price "diving", compared with the previous medical insurance catalogue, which is only updated once every seven or eight years, the introduction of policy-side medical insurance payment and negotiation forms has become a strong driving factor for the "high progress" of China's innovative drug research and development.
03
Folding the laurel and folding the halberd, a word away
Folding the laurel and folding the halberd, a word away.
At the beginning of July, pharmaceutical stocks were all green.
Previously, CDE publicly solicited opinions on the "Guidelines for Clinical Research and Development of Clinical Value-Oriented Anti-tumor Drugs" - on the day of the release of the document, the stock prices of wuXi AppTec and Tigermed, the two major CRO leaders, WuXi AppTec and Tigermed, fell in response, and a number of CRO/CMO/CDMO stocks dived, and the market was deeply shaken.
The guiding principles convey two messages: when conducting clinically controlled trials of drugs, one should try to provide the best treatment method/drug in clinical practice for subjects; the other is that new drug development should provide patients with better treatment options as the highest goal.
Market analysis generally believes that under the New Deal, the previously inefficient and low-level "pseudo-innovative drugs" will be terminated.
Although the epidemic in 2021 is still repeated, Wang Yanan still travels between different cities to recruit subjects.
"In 2009, when I first came into contact with the CRO industry, China's pharmaceutical clinical trials were still in a 2G era, and now we are already moving towards the 4G era, and we will move towards 5G with the changes in the entire environment." Wang Yanan sighed.
He believes that in the process of China's innovative drugs continue to strengthen, the drug regulatory department has cleared many obstacles for enterprises, especially after joining the ICH, supervision has become more and more professional and standardized, although the lowest level of popularization and support needs to be further strengthened, but all progress is more needed is time and necessary patience.
It is worth noting that on April 15 this year, the Drug Review Center issued the "Guiding Principles for Real-World Data for Generating Real-World Evidence (Trial)", which plans to conduct retrospective research or explore the model of real-world data to support the research and development of children's drugs for drugs that have not yet been approved for use in children worldwide, but domestic and foreign guidelines have clearly recommended drugs for children, and effectively solve factors such as long clinical trial cycles, recruitment difficulties, and high safety risks for pediatrics.
Not long ago, Su Xian, a drug evaluation center of the State Drug Administration, wrote in the magazine "China Food and Drug Administration" that the drug review center has begun to guide the rational development of enterprises in individual research and development overheating areas, and will use limited review resources on innovative drugs and urgently needed drugs with obvious clinical value.
As mentioned in the article, in 2020, the Drug Review Center opened a number of channels for accelerating the listing and registration procedures of drugs; more than 300 guiding principles have been revised in recent years; in the first half of 2021, 21 class 1 innovative drugs have been approved for listing, and the treatment field mainly involves the prevention and treatment drugs needed for emergency public health emergencies and clinical urgently needed drugs such as tumors, rare diseases and immune system diseases, many of which are products independently developed by China and have independent intellectual property rights—— The article emphasizes that guiding the differential research and development of new drugs is an important work direction of the drug evaluation center in the future.
In the eyes of the R&D personnel of a well-known CRO company, the industrialization of achievements in basic research and universities or a breakthrough point for the smoother clinical trial of new drugs in the future.
"Now affected by the collection, more and more companies are starting to develop in the direction of innovative drugs or looking for First In Class drugs." The above-mentioned R & D personnel believe that our current great difficulty lies in basic research, and the understanding and verification of drug targets are still relatively backward, which is also a place that is still lagging behind foreign countries at this stage.
In the long run, the establishment of a sound achievement transformation mechanism, a complete drug research and development ecology, the real spring of drug research and development will surely come.
After all, no one ever thought that from the approval of the first independent innovation drug ektinib hydrochloride in 2011 to whether there is a "bubble" in China's innovative drug market in 2021, China has only spent ten years.
In the next decade, can China's innovative drugs once again open up a new track of development from 0 to 1?
Do as you please, and sail with a reed.