A new study by a team of San Diego scientists suggests that serine palmitoyl transferase can be used as a metabolic response "switch" to reduce tumor growth, and the team published their findings In Nature on Aug. 12.
< h1 class="pgc-h-arrow-right" > findings</h1>
By restricting the amino acids serine and glycine in the diet, or targeting the serine synthase phosphoglycerol dehydrogenase by pharmacological action, the team induced tumor cells to produce toxic lipids, thereby slowing down the cancer process in mice. Further research is needed to determine how to translate the method into patients.
< h1 class="pgc-h-arrow-right" > research background</h1>
Over the past decade, researchers have learned that removing the amino acids serine and glycine from animal diets slows the growth of certain tumors. However, most research teams have focused on how these diets affect epigenetics, DNA metabolism, and antioxidant activity. In contrast, researchers from the University of California, San Diego and the Sark Institute for Biology found that these interventions had a huge impact on tumor lipids, especially on the cell surface.
Christian Metello, professor of bioengineering at the Jacobs School of Engineering at the University of California, San Diego, said: "Our work highlights the complexity of metabolism and the importance of understanding physiology across multiple biochemical pathways when considering this metabolic therapy. ”
<h1 class="pgc-h-arrow-right" > mechanism of action</h1>
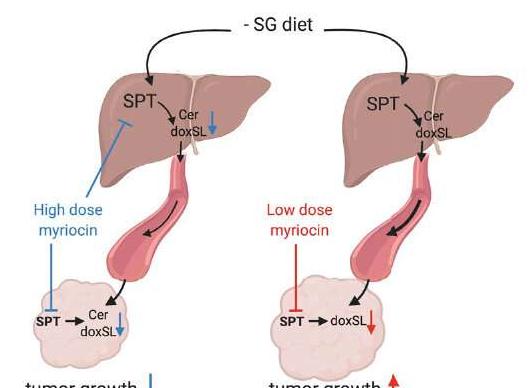
In this case, serine metabolism is the focus of the researchers. Serine palmitoyl transferase (SPT) typically uses serine to make fat molecules called sphingolipids, which are essential for cellular function. However, if serine levels are low, the enzyme can act "hybridly" and use other amino acids such as alanine, resulting in toxic deoxysphingolipids.
< h1 class="pgc-h-arrow-right" > research method</h1>
The team decided on this direction after examining the affinity of certain enzymes with serine and comparing them to the concentration of serine in tumors. These levels are called km or michaelis constants, and the numbers point to SPT and sphingolipids.
These toxic deoxysphingheasters are most effective at reducing cell growth under "anchor-dependent" conditions, in which case the cells cannot easily adhere to surfaces that better mimic tumor growth in vivo. In order to better understand the mechanism by which deoxysphingolipids are toxic to cancer cells and their effects on the nervous system, further research is necessary.
In the Nature study, the team fed xenografted model mice a diet low in serine and glycine. They observed that the conversion of SPT to alanine produced toxic deoxysphingolipids instead of normal sphingolipids. In addition, the researchers used the amino acid antibiotic myristic hormone to inhibit SPT and deoxysphingosine synthesis in mice on a low serine and glycine diet, and found that tumor growth improved.
Metallo notes that long-term deprivation of serine organisms can lead to neuropathy and eye disease. Last year, he co-led an international team that identified reduced serine levels and the buildup of deoxysphingolipids as a rare form of macular disease (called macular capillary dilation type 2) or MacTel. The study was published in the New England Journal of Medicine. However, serine restriction or drug therapy for tumor treatment will not require prolonged treatment of neuropathy in induced animals or age-related diseases.