Calcium is a class of commonly used clinical drugs, which can not only be used for the prevention and treatment of rickets in children and osteoporosis in the elderly, but also widely used in the treatment of allergic diseases, kidney stones, chronic kidney disease minerals and bone abnormalities, hypocalcemia and other diseases.
1. Calcium is commonly used in clinical practice
At present, calcium preparations on the market can be divided into four categories: inorganic calcium, organic calcium, natural calcium and new calcium.
Natural calcium (oyster calcium carbonate) is a mixture of calcium carbonate and calcium hydroxide prepared by high temperature calcination and conversion of oyster shells, which is essentially inorganic calcium and has no other biological activity.
The new calcium (compound amino acid chelated calcium) is a compound preparation composed of amino acid chelated calcium, magnesium, zinc, copper, manganese, vanadium, silicon, boron, dibasic calcium phosphate, calcium ascorbate, and vitamin D3.
Note that many calcium salts are hydrates.
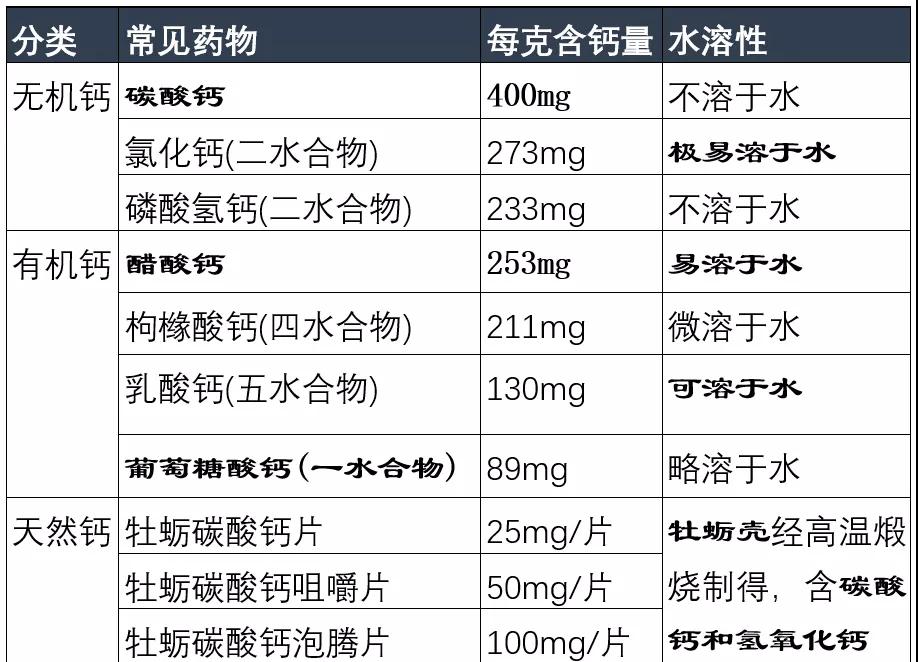
Second, when selecting calcium agents, it is necessary to pay attention to "water solubility"
The water-soluble nature of calcium salts is not related to gastrointestinal stimulation, and the highly soluble calcium chloride has the greatest stimulation of the gastrointestinal tract.
Calcium carbonate is insoluble in water and only has a high solubility rate in gastric acid, so patients with gastric acid deficiency should not be selected. Patients with gastric acid deficiency may consider using calcium agents with good water-soluble properties, such as calcium acetate, calcium lactate, calcium gluconate, calcium citrate, etc.
Refractory calcium salts are generally considered to be more likely to cause constipation.
The calcium and a variety of trace elements in the compound amino acid chelated calcium form chelates with amino acids to avoid the metal ions combining with carbonate, phosphate or hydroxide ions to form a precipitate. Therefore, it will not cause adverse reactions such as constipation.
Third, when selecting calcium agents, it is necessary to pay attention to "acid root"
1. Special adverse reactions
Calcium carbonate neutralizes with stomach acid to produce carbon dioxide, which can cause belching.
2. Effect on aluminum absorption
Animal experiments have found that calcium citrate and calcium acetate as calcium supplements may promote the absorption of aluminum and lead, which is not conducive to the excretion of aluminum and lead from the body, and has a negative effect on maintaining the health of bones and nervous system.
Relatively speaking, calcium carbonate may be safer as a calcium supplement.
3. Treatment of hyperphosphatemia
Calcium carbonate and calcium acetate can be combined with phosphate in food in the digestive tract to form calcium phosphate that is not easily absorbed, reducing the absorption of phosphorus, and can be used for the treatment of hyperphosphatemia.
Compared with calcium carbonate, calcium acetate binds to phosphorus in food twice as much as calcium carbonate, but calcium absorption is lower than calcium carbonate, and is often used clinically for the treatment of hyperphosphatemia caused by chronic renal failure.
Tips:
2020 Medical Insurance Catalog: Calcium acetate oral regular-release dosage form, limiting hyperphosphatemia due to chronic renal failure.
4. Treatment of allergic diseases
Calcium ions can improve the permeability of cell membranes, increase the density of capillaries, reduce exudation, and have a certain anti-allergic effect.
Calcium gluconate is often used as an adjunctive treatment for allergic diseases.
Fourth, the best time to take calcium
It is related to the purpose of the drug.
1. Osteoporosis: oral administration 1 to 1.5 hours after meals, food can promote gastric acid secretion, is conducive to the dissolution and absorption of calcium.
2. Hyperphosphatemia, hyperoxalic aciduria: oral administration during meals or immediately after meals, calcium can be more effectively combined with phosphorus and oxalic acid in food, reducing the absorption of phosphorus and oxalic acid.
Calcium is absorbed better than once taken several times a day, and a one-time overdose of calcium may increase the risk of cardiovascular events.
appendix:
Adults can consume about 400 mg of elemental calcium per day through diet, which is about 50% of the recommended amount.