For a long time, organic solar cells (OSCs) have become a hot spot in the field of solar cell research because of their advantages of light weight, strong flexibility, high flexibility, low cost, and large area preparation. The development of high-performance, environmentally friendly OSCs has always been a relentless goal pursued by researchers, and with the advent of non-fullerene receptor molecules (NFAs), the photoelectric conversion efficiency (PCE) of OSCs is now more than 18%, which is close to commercial application. NFAs show greater advantages over conventional fullerene receptors, including easier synthesis and purification, a wide range of absorption of sunlight, easy modification of molecular structures, and tunable electronic energy. End group halide of NFAs is a common strategy mainly used to adjust molecular energy levels and optical bandgaps, so in order to enhance molecular redshift absorption capacity and improve charge transfer, researchers often use end group fluorination and chlorination methods, but bromination currently receives less attention. In fact, the low cost of bromination, simple synthesis, easy operation, and also conducive to the modification of subsequent reactions, has an important role in the synthesis of high-performance NFAs.
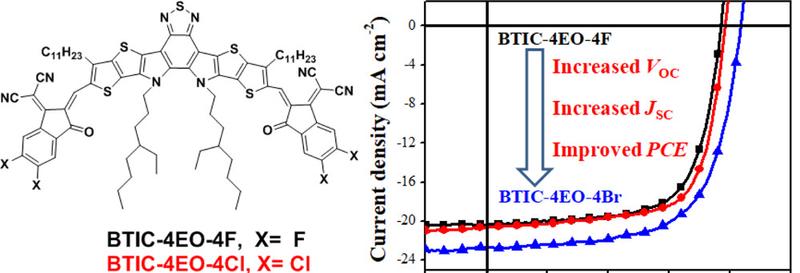
In order to systematically study the effects of end group fluorination, chlorination and bromination of NFAs on the performance of OSCs, the research team of Professor He Feng of Southern University of Science and Technology recently designed and synthesized a series of NFAs: BTIC-4EO-4F, BTIC-4EO-4Cl and BTIC-4EO-4Br. All three NFAs have a fused benzothiadiazole unit as the core, and fluorinated, chlorinated and brominated end groups as the terminal units. The results show that end group halide can effectively enhance the intramolecular electron transfer and broaden the absorption spectrum of the molecule, so that the final molecule has a narrow band gap and high electron mobility. The researchers also found that BTIC-4EO-4Br exhibited lower LUMO levels, smaller band gaps, and stronger intermolecular interactions than BTIC-4EO-4F and BTIC-4EO-4Cl because bromine atoms were more capable of enhancing intramolecular charge transfer.
The researchers then blended three NFAs with polymer donor PBDB-TF to prepare three OSCs devices and tested the corresponding photovoltaic performance separately. The results show that the PBDB-TF-BTIC-4EO-4F-based OSC device has a PCE of 10.64%, an open-circuit voltage (VOC) of 0.77 V, and a short-circuit current density (JSC) of 20.01 mA cm−2. Due to the redshift of BTIC-4EO-4Cl spectral absorption, the PCE of OSC devices based on PBDB-TF: BTIC-4EO-4Cl increased to 11.29%, and VOC, JSC also increased to 0.78 V and 20.59 mA cm−2, respectively. However, OSC devices based on PBDB-TF:BTIC-4EO-4Br reached a maximum of 12.41% due to the significantly improved VOC (0.84 V) and JSC (22.78 mA cm−2) while having minimal energy loss. In addition, the device blend film shows better phase separation size, a highly ordered structure, and a stronger stacking layer.
In conclusion, this study not only shows that end-group bromide is an effective strategy to enhance the charge transfer capacity of NFAs and improve the photovoltaic performance of final OSCs, but also provides new ideas for subsequent performance improvements of related NFAs. The results are now published in ACS Applied Materials & Interfaces, entitled "Effects of Halogenated End Groups on the Performance of Nonfullerene Acceptors".
Literature links: https://dx.doi.org/10.1021/acsami.0c17598
Materials involved in the text:
PBDB-TF(PM6):1802013-83-7,IC2F:2083617-82-5,IC2Cl:2197167-50-1,IC2Br