On the evening of the 18th, some media reported that since May 13, all districts in Shanghai have begun to supply recombinant new coronavirus vaccines (type 5 adenovirus vectors) produced by CanSino Biologics. Only one dose of the vaccine is given. It is understood that in addition to Shanghai, the vaccine has also been launched in Beijing, Tianjin, Zhejiang, Henan, Anhui and other places.
As soon as the news came out, the capital market responded enthusiastically. As of press time, CanSino rose more than 10% in the afternoon, and the stock price was reported at 562 yuan, with a turnover of 1.187 billion yuan.
A race against time A number of biological companies have been approved for the new crown vaccine
According to the announcement of CanSino, the Ad5-nCoV (adenovirus vector type 5) vaccine was developed by the team of academician Chen Wei of the Institute of Biological Engineering of the Academy of Military Sciences of the People's Liberation Army Academy of Military Sciences of Chinese, and the adenovirus vector vaccine was the first clinical study in the world on March 16, 2020. On February 25 this year, the vaccine was approved by the State Drug Administration for conditional marketing in China. It is understood that Ad5-nCoV only needs to be vaccinated in one dose, suitable for people aged 18 and above, and can be stored and transported in an environment of 2 °C-8 °C. Previous Phase III clinical trial data showed that after 28 days of a single dose of Ad5-nCoV, the total protective efficacy of the vaccine for all symptoms was 65.28%, and the protective efficacy for severe disease was 90.07%.
According to the news from Shanghai Disease Control, those who have received 1 dose of inactivated vaccine are recommended to use inactivated vaccine to complete the whole process of vaccination; those who have been vaccinated with 2 doses of inactivated vaccine are not recommended to use adenovirus vaccine to strengthen. Given that domestic inactivated vaccines have begun to be mass-vaccinated, how much market share will be left for CanSino Ad5-nCoV? This is a matter of great concern to many investors. In the future, can the vaccine produced by CanSino go "to sea"? As of press time, Cansino has not replied to the Aoyi reporter.
In addition, earlier, CanSino Bio issued an announcement on the Hong Kong Stock Exchange that its inhalation recombinant novel coronavirus vaccine had obtained the approval of the State Drug Administration for drug clinical trials. Yu Xuefeng, co-founder, chairman and CEO of CanSino Biologics, revealed that the vaccine induces triple protection of humoral immunity, cellular immunity and mucosal immunity, which is better and more convenient to use. "Inhaled vaccines can achieve the same effect as an injection in human cellular immunity and antibody immunity with a dose of 1/5. Inhalation can achieve significant cost savings and is of great significance for us to expand our production capacity. ”
Statistics from Aoyi news reporters found that 6 new crown vaccines have been approved for marketing or emergency use in the domestic market, and vaccines are divided into three categories with different technical routes: one is inactivated vaccines; the other is adenovirus vector vaccines; and the third is recombinant new coronavirus vaccines (CHO cells). The representative enterprises are: Kexing Zhongwei, Sinopharm Beijing Institute, Sinopharm Wuhan Institute, Kangxino, Zhifei Biological and Kangtai Biological. In addition, the mRNA covid-19 vaccine jointly developed by Fosun Pharma and BioNTech in Germany has entered the countdown to domestic marketing.
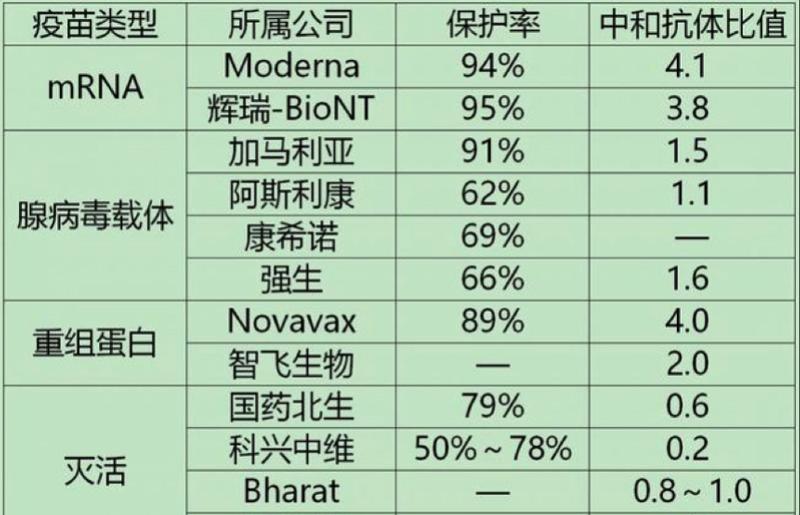
On May 14, Kangtai Bio issued an announcement that the new crown inactivated vaccine developed by the company was recommended by the National Health Commission, and the State Food and Drug Administration organized demonstration and consented to emergency use. It is understood that as early as February 2020, Kangtai Bio began to start vaccine research and development, and obtained clinical trial approval from the State Food and Drug Administration in September of the same year.
It is worth noting that Kangtai Bio has also laid out two other technical routes for the development of new crown vaccines. In August last year, Kangtai Bio signed a "licensing agreement" with AstraZeneca in the United Kingdom to introduce its new ChAdOx1 adenovirus vector vaccine in cooperation with Oxford University, and obtained an exclusive license for research and development, production and commercialization in China. Based on the progress of R&D, if it goes well, it is expected to be approved in the third quarter of 2021 and have a production capacity of 200 million doses by the end of 2021 to meet the needs of the domestic market.
Citic Construction Investment Research Report previously analyzed that the new crown vaccine will enter the commercial centralized cashing stage in 2021, according to the data of the Cold Chain Committee of the China Federation of Things, the global new crown vaccine market size can reach 880 billion yuan.
Increasing production capacity under mass inoculation conditions is key
At present, there is still a lot of room for improvement in the domestic COVID-19 vaccination rate. According to data from the Health Commission, as of May 19, there were about 440 million doses of COVID-19 vaccination in China. According to soochow Securities Research Report, the domestic vaccination rate is less than 20%, while the United States has reached more than 40%. As a number of approved new crown vaccine manufacturers, they are expected to increase their volume rapidly and become a performance growth point.
As the sixth domestic manufacturer of the new crown vaccine approved for emergency use, Kangtai Bio said that the vaccine research and development and industrialization work is progressing smoothly, and the supporting production workshop located in Nanshan District of Shenzhen has completed construction and is fully put into production. According to the announcement of Kangtai Biology, its Baiwangxin emergency engineering construction project is mainly used for the production of new crown inactivated vaccines, with a project operation period of 10 years and a design capacity of 200 million doses/year. Considering the flexibility of production capacity, Soochow Securities Research Report expects that its actual production capacity will far exceed the design capacity.
On May 17, CanSino announced in Hong Kong that in view of the global demand after the commercialization of Ad5-nCoV (adenovirus vector type 5), it is planned to use Shanghai Pharmaceutical CanSino as its production base in Shanghai for the production and supply of Ad5-nCoV, with an annual production capacity of not less than 200 million doses. According to CanSino's latest financial report, the company is also actively seeking partners to ensure the production capacity and subsequent supply of new crown vaccines. With the continuous promotion of the production capacity construction of Shanghai Pharmaceutical CanSino and the continuous optimization of the process, the overall production capacity of CanSino Bio's new crown vaccine will be rapidly improved, and it is expected to accelerate the annual production capacity of 500 million to 700 million doses.
Zhou Feng, director of the recombinant new crown vaccine (CHO cell) workshop of the production management department of Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd., told the media that the vaccine production capacity has stabilized. "The company has 1 stock solution line, 4 formulation lines and 7 packaging lines, and can produce 2,000 doses of recombinant COVID-19 vaccine per minute; more than 1 million doses per day." It is reported that since the approval of the country's first recombinant new crown vaccine (CHO cells) in March this year, Anhui Zhifeilong Kema Biopharmaceutical Co., Ltd. has produced more than 100 million doses of vaccines. "If all goes well, we will produce 500 million doses of vaccine this year."
Vaccine companies that were approved for listing earlier are more confident. Earlier, Liu Peicheng, spokesman for Kexing Holding Biotechnology Co., Ltd., said that Kexing currently has at least 6 million doses of vaccines being produced every day. It is understood that Kexing has three new crown vaccine production workshops put into operation on April 1, with a total annual production capacity of more than 2 billion doses. According to incomplete statistics, the total number of COVID-19 inactivated vaccines vaccinated by Kexing China in the world has exceeded 250 million doses.
Yang Xiaoming, chairman of Sinopharm China Biologics, revealed that the high-grade bio-safety production workshops for the new crown inactivated vaccine built by China Biologics in Beijing and Wuhan have been put into large-scale production, and the second-phase production capacity is undergoing simulation verification of the new workshop, and the production capacity is expected to reach more than 1 billion doses in 2021.
According to Fosun Pharma's earlier announcement, it and BioNTech will say that Fosun Pharma, a holding subsidiary, intends to invest in a joint venture with BioNTech to realize the localization and commercialization of mRNA COVID-19 vaccine products. The joint venture will have an annual production capacity of 1 billion doses of mRNA covid-19 vaccine.
From "catching up" to "overtaking", domestic vaccines lead the trend of "going to sea"
In the new crown epidemic, the research and development and listing progress of new crown vaccines on many technical routes of Chinese enterprises are in the leading position in the world. At present, There are many new coronavirus vaccines in China that have been put into use for immunization at home and abroad, and have taken the lead in entering the international market, becoming a successful case of China's vaccine catching up with and overtaking development.
According to the Beijing Capital Securities Research Report, the vaccine industry has extremely high barriers to entry, and China's vaccine head enterprises will have heavy varieties listed in the next few years, and the second quarter of this year will usher in the considerable performance contribution brought by the new crown vaccine. The NEW CROWN vaccine will become the world's largest variety in annual vaccination volume and sales (more than 13-valent pneumonia conjugate vaccine and HPV vaccine), and it is also a rare opportunity for China's vaccine industry to go overseas.
"Our COVID-19 vaccine is already in short supply. Since the COVID-19 epidemic has no borders, any vaccine will become a global public good after it is approved for marketing, so it is now a golden period for every company involved in the development and production of the new crown vaccine. Feng Duojia, president of the China Vaccine Industry Association, believes that the domestic new crown vaccine is gradually entering the harvest period, and the main contradiction in the field of new crown vaccine will be transformed from vaccine research and development to vaccine industrialization.
The global spread of COVID-19 has given Chinese vaccine manufacturers the opportunity to enter overseas markets. At present, Malaysia, Turkey, the Philippines, Mexico and other countries have ordered Chinese vaccines. Countries such as Indonesia, Pakistan and Mexico have signed agreements with China to produce vaccines. The large-scale overseas of domestic new crown vaccines may become the vanguard of the fierce international vaccine market.
Aoyi news reporter Lin Shiyan