JESSLYN SHIELDS
Bohr's atomic model
You can search for pictures of atoms on the internet, even if no one has actually seen them. But thanks to the work of a bunch of different scientists like Danish physicist Niels Bohr, we've estimated the appearance of individual atoms.
Atoms are the basis of matter, and the individual atoms of any element are the most basic entities in nature, still obeying the physical rules that we can observe in our daily lives (the subatomic particles that make up atoms have their own special rules). Scientists suspect that atoms exist for a long time before they can conceptualize their structure — even the ancient Greeks believed that problems with the universe were made up of so small components that they couldn't be broken down into anything smaller, and they called these fundamental units atoms, meaning "undivided." By the end of the 19th century, it was already known that chemicals could be broken down into very small atoms, and that atoms of different elements had predictable weights.
But then, in 1897, the British physicist J.J. Thomson discovered that the smallest thing that existed was the electron—the negatively charged particle inside atoms that everyone had thought were completely indivisible for most of the century. Thomson only assumed that electrons existed, but he couldn't calculate exactly how electrons were embedded in atoms. His best guess is the "ionic pudding model," which describes atoms as positively charged pies scattered with negatively charged regions, like fruits scattered in old-fashioned desserts.
Harvard chemist Dudley Herschbach said: "Electrons were found to be negatively charged, of the same mass compared to atoms, and very small. He was awarded the Nobel Prize in Chemistry in 1986 for his "contributions to the kinetics of fundamental processes in chemistry." ”。“ Ernest Rutherford discovered the nucleus in 1911. The nucleus is positively charged, has a variety of masses, but is much larger than an electron, but small in size. ”
<h1 class= "pgc-h-arrow-right" > a huge leap forward</h1>
Niels Bohr, a student of Rutherford, cleverly took over his mentor's plan to interpret atomic structures in 1912. It took him only a year to come up with a working model of the hydrogen atom.
Niels Henrik Bohr (1885-1962) was a Danish physicist who developed atomic models and won the 1922 Nobel Prize in Physics
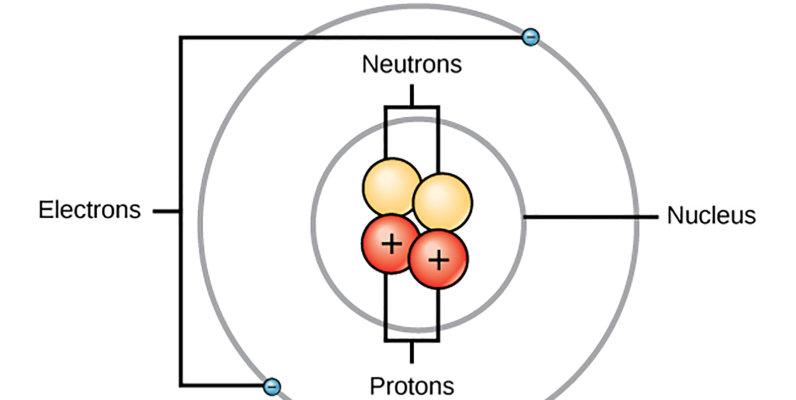
Hirschbach said: "Bohr's model of the hydrogen atom, which Bohr established in 1913, has circular electron orbits around protons, just like the Earth orbits the Sun. "Bohr employs a simple and regular pattern for the spectrum of hydrogen atoms, which John Balmer discovered in 1885." He also took advantage of the idea of quantum concepts discovered by Max Planck in 1900. ”
In 1913, the Bohr model made a huge leap forward because it integrated the features of nascent quantum mechanics into the description of atoms and molecules. That year, he published three papers on the composition of atoms and molecules: the first and most famous on the hydrogen atom, and the other two describing elements with more electrons within the framework of his model. The model he proposed for the hydrogen atom causes electrons to move around the nucleus, but only in special orbits with different energy levels. Bohr hypothesized that electrons emit light when they transition from high-energy orbitals to low-energy orbitals, which is why hydrogen emits light in glass tubes. He was right about hydrogen, but there was something wrong with his model.
"The model cannot predict the correct values for the ground state energy of multi-electron atoms and the binding energy of molecules, even for the simplest 2-electron systems, such as helium atoms or hydrogen molecules," said Anatoly Svidzinsky, a professor at Texas A&M's Institute of Quantum Science and Engineering, in an email. So, back in 1913, it was clear that bohr's model wasn't quite right. Even for hydrogen atoms, the Bohr model incorrectly predicts that the ground state of the atom has non-zero orbital angular momentum. ”
< h1 class = "pgc-h-arrow-right" > the 1922 Nobel Prize</h1>
If you're not a quantum physicist, that certainly doesn't mean much to you. However, Bohr's model was fast-tracked by the 1922 Nobel Prize in Physics. But while Bohr is cementing his reputation in the physics community, scientists are improving his model:
"The Bohr model of the hydrogen atom was improved in 1916 by Arnold Sommerfeld," Herschbach said. He found an elliptical orbit that has spectral lines close to those of a circular orbit. The Bohr-Sommerfeld model of the hydrogen atom is fundamental, but quantification and relativity become the main aspects. ”
The Sommerfeld model of the semi-classical electron orbit was improved on the Bohr model in 1916
The Sommerfeld model of the semi-classical electron orbit was improved on the Bohr model in 1916.
Between 1925 and 1928, Werner Heisenberg, Max Byrne, Wolfgang Pauly, Owen Schrodinger, and Paul Dirac proposed these aspects far beyond Bohr's atomic model, but he is by far the most recognized atomic model. The atomic model of quantum physics makes us look less like the sun surrounded by electron planets and more like modern art. We are likely still using the Bohr model because it introduces the concept of atoms well.
Svidzinsky said: "In 1913, Bohr's model proved that quantification is the right way to describe the microscopic world. "Thus, the Bohr model points scientists in the direction of finding and stimulates further developments in quantum mechanics." If you know the path, then sooner or later you will find the right way to fix the problem. One can think of the Bohr model as one of them. Signs in the direction of entering the quantum world along the hiking trail. ”