directory
1, nitrosamine impurities introduction ..............................
2. Follow-up of various regulatory agencies of nitrosamine impurities
3. Summary of regulations and guidelines............
4, the provisional limit ... ...... ...... ...... ......
5, detection methods...............
6, the training of regulators ... ...... ......
7, reference sources .....................
Introduction to nitrosamine impurities
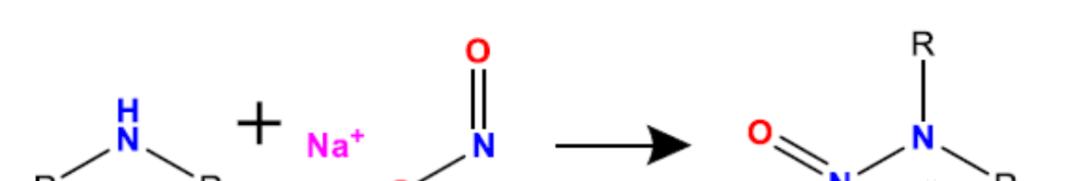
The term nitrosamines refers to a class of compounds that contain nitroso structures connected to amine groups (R1N(-R2)-N=O), as shown above. These compounds can be produced by nitrosamineization reactions of amines (secondary, tertiary or quaternary amines) and nitrites (nitrites in an acidic environment).
N-Nitrosodimethylamine (NDMA) and other nitrosamines are common contaminants found in foods, beverages, cosmetics, water, tobacco products, and other daily consumables, with low levels (ppm).
There are many types of nitrosamine impurities identified at present, which can refer to some lists issued by the FDA and EMA, which are not exhaustive and will be updated as needed if applicable regulatory structures apply.
Nitrosamine impurities followed up by regulatory agencies
- FDA: Information about Nitrosamine Impurities in Medications
https://www.fda.gov/drugs/drug-safety-and-availability/information-about-nitrosamine-impurities-medications
The FDA has been updating the relevant information about nitrosamine impurities since the Huahai Valsartan incident, the FDA has a special website to summarize the relevant information, the home page of the website has the latest information, what you should know about nitrosamine impurities, and other resources provided.
Regarding the latest information section, there are currently 5 categories of affected products (see the figure below), and each module has a lot of information and is very rich in content. FDA is listed in chronological order of publication and includes:
- The analytical methods of related impurities are provided for reference by regulators and manufacturers (fda specifically indicates that reference to these methods or method verification should be done);
- Recall information for related products;
- Provisional limits of each nitrosamine impurity;
- Warning letters to manufacturers due to nitrosamine regulatory issues, etc.
B. EMA: Human Regulatory- Nitrosamine impurities
https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/nitrosamine-impurities
The European Medicines Agency (EMA) assesses the risks of nitrosamine formation or presence in the production of pharmaceutical products for human use and provides guidance to marketing authorization holders. EMA in the human medication guide module increased the relevant update content of nitrosamine impurities, similar to the FDA, EMA will also publish all the content on the website for your reference, and the FDA's presentation is different, the top of the website has a directory, you can quickly find what you want to find according to the directory.
Summary of regulations and guidelines
The FDA and EMA guidelines for nitrosamine impurities can be found on the above page, and here I have selected some for brief introduction for your reference.
Tentative limits
Detection method
Training for regulators
The above summary is mainly the FDA and EMA related resources on nitrosamine impurities, a total of everyone to find and learn
Reference Sources
[1] FDA, https://www.fda.gov/
[2] SBIA, https://sbiaevents.com/
[3] EMA, https://www.ema.europa.eu/en
[4] EDQM,https://www.edqm.eu/en
[5] NMPA, https://www.nmpa.gov.cn/
Article from the registration circle public number ~ welcome to pay attention to Oh ~