Original Junchen Zuo envoy Minnet
Great content
On March 3, the official website of the State Food and Drug Administration showed that the dexmedetomidine hydrochloride injection of the 3 types of generic drugs in Chia Tai Qingjiang was approved for production and regarded as overrated. According to data from the intranet, the sales of the product in China's public medical institution terminals in 2020 will be close to 4 billion yuan, an increase of 10.61% year-on-year.
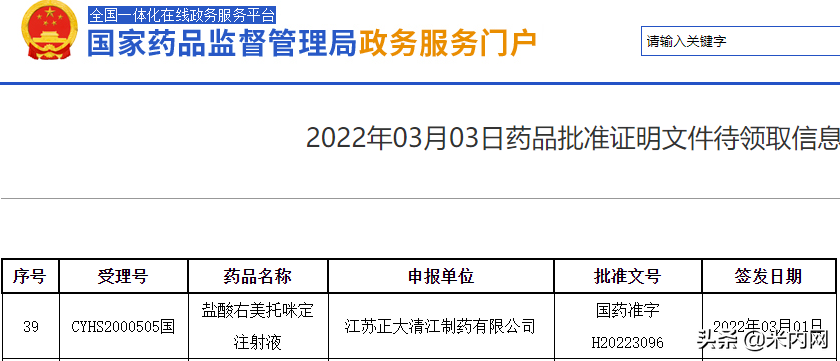
Source: The official website of the State Food and Drug Administration
Dexmedetomidine hydrochloride belongs to the alpha2-adrenoceptor agonist, which has sedative, analgesic, anxiolytic and other effects, and is clinically used for sedation during endotracheal intubation and mechanical ventilation in surgical patients undergoing general anesthesia.
At present, there are 16 domestic enterprises with dexmedetomidine hydrochloride injection production approval, intranet data show that the product in 2020 China's urban public hospitals, county-level public hospitals, urban community centers and township health centers (referred to as China's public medical institutions) terminal sales scale of nearly 4 billion yuan, an increase of 10.61%. Relying on the exclusive bid in 4+7 and expanded area collection, Yangtze River's products have increased rapidly in recent years, and currently have an exclusive market share of more than 90%.
Dexmedetomidine hydrochloride injection was partially evaluated
Source: Intranet Consistency Evaluation Database
Before Chia Tai Qingjiang, 14 domestic enterprises have passed or treated the consistency evaluation of dexmedetomidine hydrochloride injection, including Yangzijiang, Hengrui, Beite, Enhua, Nanjing Chia Tai Tianqing, etc.; in addition, nearly 10 enterprises such as Kangpu Pharmaceutical, Hebei Renhe Yikang, Chia Tai Fenghai, Minsheng Pharmaceutical and other nearly 10 enterprises have submitted supplementary applications for consistency evaluation or reported production in new classifications, and are still under review and approval.
Zhengda Qingjiang commented on the situation
Source: MED2.0 China Drug Review Database
Including dexmedetomidine hydrochloride injection, there are currently 5 varieties in Chia Tai Qingjiang that have passed or are deemed to have passed the consistency evaluation, of which glucosamine hydrochloride tablets are exclusively evaluated.
Source: Intranet database, the official website of the State Food and Drug Administration, etc
Note: Data statistics as of March 4, if there is any omission, welcome to correct!