On February 10, Lepu Bio officially opened its prospectus, and it is expected that the shares will begin trading on the Hong Kong Stock Exchange on February 23, 2022. As a spin-off company from Lepu Medical, Lepu Biotech was established in 2018 to focus on oncology drug research and development. Through the acquisition, Lepu Bio has a drug pipeline such as PD-1 monoclonal antibody and ADC. However, PD-1 monoclonal antibody and ADC are popular targets for biological drugs at present, attracting a number of pharmaceutical companies. The listing has brought financial support to Lepu Bio, but how to achieve commercialization in the future is a problem that Lepu Bio needs to think about.
Countdown to listing
From A shares to Hong Kong stocks, Lepu Bio landed on the capital market as desired. On February 10, Lepu Bio officially opened the IPO, the IPO is expected to issue 127 million shares, of which 12.688 million shares are publicly offered in Hong Kong, 114 million shares are sold internationally, and 19.031 million shares are over-allotted, and it is expected to be listed on the Hong Kong Stock Exchange on February 23.
Lepu Bio has been preparing for the market for a long time. In the second half of 2020, Lepu Bio and Haitong Securities signed a listing counseling agreement, which was filed with the Shanghai Securities Regulatory Bureau on December 17 of the same year to be listed on A-shares. In April 2021, Lepu Biotech submitted a form to the Hong Kong Stock Exchange for listing on the Main Board. After the application materials "expired" and then submitted the form, Lepu Bio finally passed the hearing. Before the IPO, Lepu Bio was favored by many well-known investment institutions such as Sunshine Life, Ping An Capital and Shanghai Biomedical Fund. After completing the 261 million yuan C round of financing, the valuation of Lepu Bio reached 10.261 billion yuan.
As a "Lepu department", Lepu Biology was spun off from Lepu Medical. At present, Lepu Bio has established a product pipeline consisting of drug candidates focusing on multiple indications for oncology treatment, but most of the products of Lepu Bio Pipeline are acquired through the acquisition of subsidiaries, licensing and joint ventures, and nearly half of its core assets are acquired. According to the prospectus, Lepu Bio's main pipeline assets include MRG003, MRG002, HX008 and LP002, as well as three key clinical stage drug candidates.
Among them, the global rights of ADC candidates MRG003 and MRG002 were acquired by Lepu Bio through the acquisition of a controlling interest in Shanghai Mei yake, and the anti-PD-1 antibody candidate HX008 and anti-PD-L1 antibody candidate LP002 were acquired by Lepu Bio through the acquisition of Taizhou Hanzhong Biomedical Co., Ltd. and Taizhou Houde Aoke Technology Co., Ltd.
In this listing, 68.5% of the funds raised by Lepu Biotech will be used for investment in core products, 15.8% for the acquisition of potential technologies and assets and the expansion of drug candidate pipelines, 6.3% for major clinical stage drug candidates and major pre-clinical drug candidates, and the remaining 9.4% for general use of the company.
In response to the planning and other issues after the listing in Hong Kong, the reporter contacted Lepu Biology, but as of press time, no more replies have been received.
Not yet commercialized
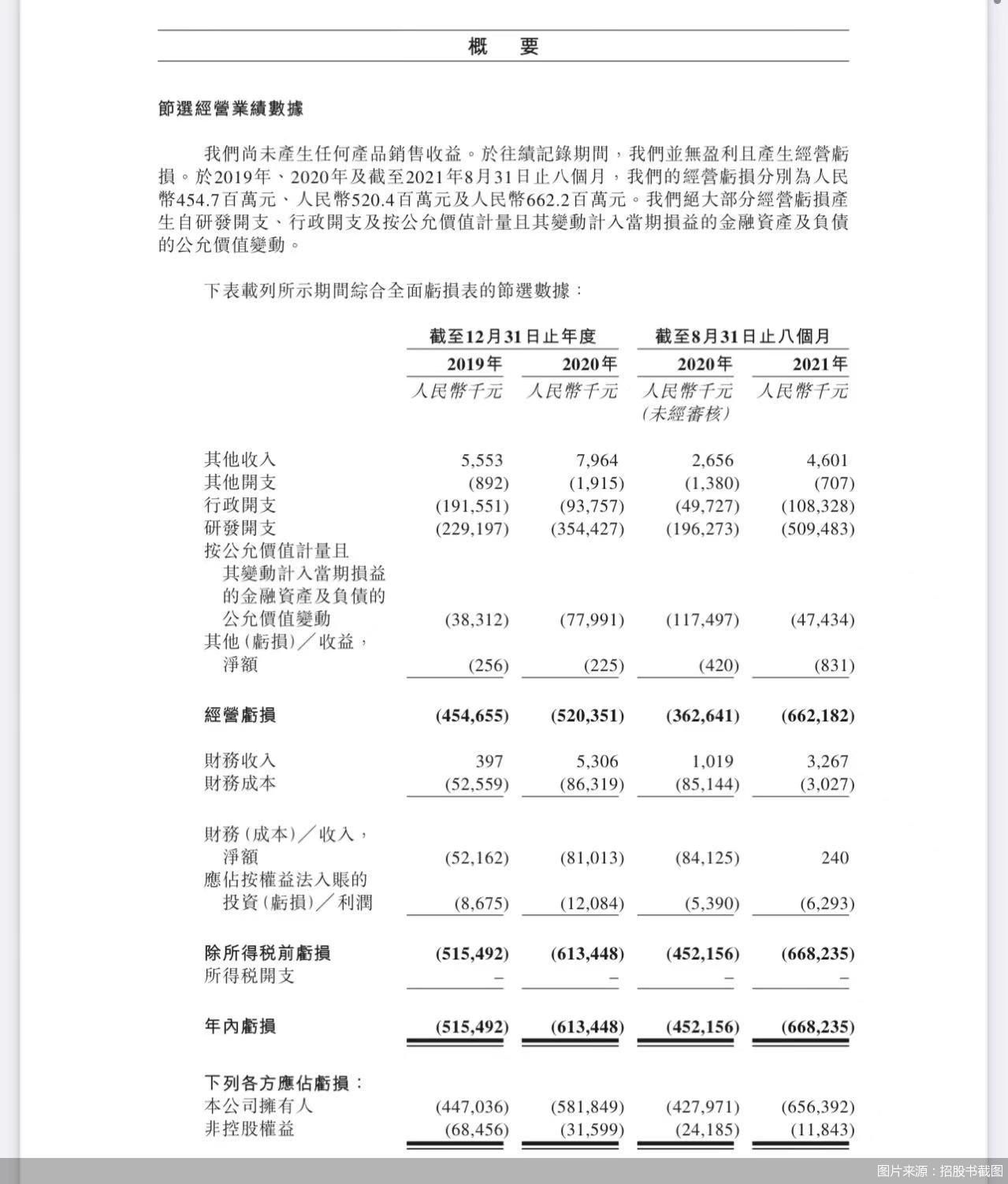
Although it has entered the domestic hot biological drug track through acquisition, under the giant lining, Lepu Biology still has a long way to go to achieve profitability.
Since its products have not yet been listed for sale, Lepu Bio is still in a state of loss. In 2020, the net loss of Lepu Bio increased by 19% from 516 million yuan in 2019 to 613 million yuan, and the net loss in two years exceeded 1 billion yuan. Lepu Bio explained in the prospectus that it was mainly due to the increase in research and development expenditure.
At present, Lepu Bio is expected to take the lead in commercialization as PD-1 monoclonal antibody product (HX008). In July 2021, Lepu Bio's HX008 listing application was officially accepted by the State Drug Administration. But how much Lepu Bio can split the pie from this market is still unknown. After the medical insurance negotiations, the annual treatment cost of PD-1 monoclonal antibody dropped to 50,000 yuan / year, and going overseas to expand the market is one of the current coping strategies of various enterprises.
Li Yan, an investment analyst in the pharmaceutical industry, believes that the remaining unlisted PD-1 monoclonal antibody products want to enter medical insurance, and the pricing can only be lower than the current price of entering medical insurance. An executive of a PD-1 monoclonal antibody manufacturer once said that after four PD-1s entered the medical insurance in 2020, the space left by this market for latecomers has been very small, and at most one can survive. According to the data, there are currently 6 PD-1 monoclonal antibody products that have been approved for marketing in China, involving non-small cell lung cancer, head and neck cancer, stomach cancer, esophageal cancer, melanoma and other cancers, 3 of which have applied for marketing, and 7 of which are in the clinical stage of Phase III.
In addition, ADC is also a hot domestic research and development track. According to incomplete statistics, there are currently 38 ADC drugs in the mainland, of which the HER2 track is the most crowded, with a total of 13 products, accounting for about 34% of the domestic ADC pipeline. MRG002, the core product of Lepu Biologics, is a HER2-targeted ADC drug and is currently in phase II clinical trials.
However, Lepu Bio has confidence in the commercialization of the company. According to the prospectus, Lepu Bio plans to establish a commercialization team of 50 to 100 people by the first quarter of 2022 to engage in academic promotion, marketing and commercialization of pipeline products, and dispatch 5 to 10 commercialization team members to each province depending on the size of the local market. At the same time, Lepu Bio may seek to include some of the company's products in the national medical insurance drug list and other reimbursement plans. (Yao Qian)