Persistent high-risk HPV infection is closely related to the development of cervical cancer, and most cervical cancer cases are associated with one of 14 high-risk HPV infections, of which subtypes HPV16 and HPV18 are the cause of the disease in about 70% of cases, and vaccination is an effective method of preventing HPV infection.
At present, there are four HPV vaccines on the market in China (one domestic and three imported).Imported products are mainly based on Merck's quadrivalent and nine-valent HPV vaccines, while Merck needs to supply the global market, and domestic supply capacity is not sufficient. Wantai Bio's bivalent vaccine Cecolin is the first approved domestic HPV vaccine, with independent intellectual property rights, and the price of 329 yuan per bottle after listing is much lower than the unit price of imported similar products.
Comparison of the four HPV vaccines approved for marketing in China
Source: Huaxi Securities
According to the hpvide vaccine batch issuance of the Central Inspection Institute, a total of 20 million people in China have completed the full immunization of HPV vaccine from 2017 to 2021. At present, the number of women aged 9-45 in China is about 381 million,
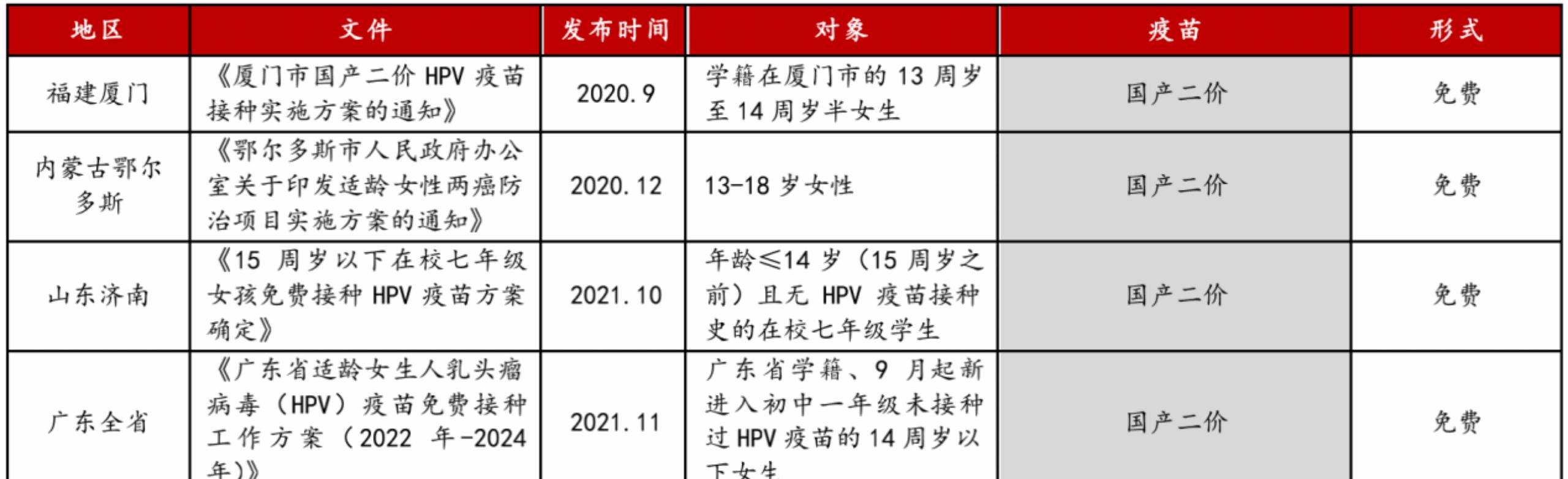
The penetration rate of HPV vaccination among women of appropriate age is only about 7%. Driven by policies, the penetration rate of HPV vaccination in China is expected to increase significantly.At present, Many places such as Xiamen in Fujian, Jinan in Shandong, Guangdong Province, Chengdu in Sichuan and other places have introduced preferential policies for HPV vaccination for women of appropriate age, and it is expected that more provinces and cities will carry out preferential policies for HPV vaccination in the future. Some local governments pay for domestic HPV vaccines, which further promotes the popularization of HPV vaccination and increases the public's awareness of the harm of cervical cancer and HPV vaccination.
In terms of competition pattern, Merck's 4/9 valent vaccine (Zhifei Biological Agent) dominates domestic HPV.
In 2017, Zhifei Bio signed a strategic agreement with Merck for the first time to exclusively represent its quadrivalent HPV vaccine, and in 2018, the two sides signed a supplementary agreement, Zhifei exclusively represented the newly listed nine-valent HPV vaccine, and in 2020, the renewal agency rights of the two parties were extended to 2023H1. 2018/2019/2020 (January-September) The company's agent product procurement amount was 3.071/60.89/7.213 billion yuan.
Zhifei Bio's renewal with Merck
Source: Debon Securities
From the perspective of batch issuanceThe batch issuance volume of 4-valent HPV vaccine in 17/18/19/20 was 34.8/380/554/721 million, respectively, and the batch issuance volume of 9-valent HPV vaccine in 18/19/20 was 122/332/507 million, showing a rapid growth trend in recent years. For Zhifei, the sufficient cash flow created through the agency products, the amount of investment in research and development of self-developed products and the speed of market promotion have been accelerated.
2017-2021Q1 Domestic HPV Vaccine Batch Issuance (10,000 Units)
In the current situation of insufficient production capacity of quadrivalent and nine-valent HPV vaccines and the government's promotion of bivalent HPV vaccines for young women, bivalent HPV vaccines still have a lot of market space.
Wantai divalent HPV vaccine was officially launched in May 2020, and about 2.1 million bottles were sold in only about half a year. According to the 2021 batch issuance data of the Central Inspection Institute, it is expected that the company's bivalent HPV vaccine batch will issue more than 10 million in 2021, taking into account the shortage of HPV vaccines in China.
It is expected that the number of issuances in 2021 will be close to 10 million, far exceeding market expectations. Compared with the bivalent HPV vaccine of imported GSKThe Wantai divalent HPV vaccine not only has the advantage of price advantage and the "two-shot method" of the low age group, but also the protective efficacy of the Wantai divalent HPV vaccine is higher than that of GSK products in preventing cancerous degeneration and persistent infection.
In terms of production capacityIn the first half of 2021, the new pre-filled syringe product line of Wantai bivalent HPV vaccine was approved, and the comprehensive production capacity of bivalent HPV vaccine was increased to 20 million bottles/year. In July 2021, the company's production scale of the preparation in the form of vials was enlarged, and it was approved by the State Food and Drug Administration
The total production capacity of the company's divalent HPV vaccine reached 30 million bottles/year. confront HPV Vaccine cake, many manufacturers are actively layout.At present, there are 13 kinds of HPV series products in China that have entered the clinical research stage, of which the professional review, clinical on-site inspection and production site inspection of Watson Biological Divalent HPV vaccine have been completed, and it is expected to be listed and sold next year; the HPV nine-valent of Wantai Biological, Bowei Biological, Kangle Guardian and Anyusheng/Ruike, Bowei Biological, Chengdu HPV Quadvalent, Zerun Biological HPV Divalent and Xiaojiang Biological/Tiande Pharmaceutical/Kangle Guard HPV Trivalent have entered the clinical phase III. and are expected to be listed for sale in 22-23 years.
Source: Northeast Securities
On the whole, in the short term, Merck 4/9-valent vaccine will continue to dominate the market exclusively, and Wantai will dominate the 2-valent vaccine market.At present, the penetration rate of 7% is only the beginning of the domestic HPV vaccine penetration rate, and in the future, with sufficient supply and the approval of male indications for domestic HPV vaccine in the future, domestic HPV vaccine sales will increase explosively. If calculated according to the penetration rate of 30%, 45% and 60% of women of the appropriate age, the current domestic HPV vaccine still needs 2.8, 45, and 620 million, and the current domestic HPV vaccine market is far from reaching the peak.