The first law of thermodynamics
ΔU=Q+W (thermodynamic energy is only the temperature function U=f(T), the change value is independent of the change path)
Isobaric volume work: W= -p(V2-V1) → dU=dQ – pdV
Quasi-static process: The expansion and compression process with infinitely small internal and external pressure is a quasi-static process, and the system does the greatest work in the expansion process, and the environment does the least work on the system during the compression process. The values are equal and the signs are opposite.
Enthalpy: H=U+pV; ΔH=Qp Derivation Process: U2-U1=Qp-p(V2-V1) → Qp=(U2+pV2)-(U1+pV1) Significance: The heat absorbed by the system during isobaric process is all used to increase enthalpy. Enthalpy, like thermodynamic energy, is only a function of temperature and is independent of p and V. In the isometry process, ΔU=Qv is calculated as ΔH=Qv-pΔV
ΔrHθm: In the standard state (100Kpa, 298.15K), the reaction with a reaction progress of 1 mol is called standard molar enthalpy, expressed in ΔrHθm(T).
ΔfHθm: At a standard pressure of 100Kpa, 298.15K), the enthalpy of 1mol of pure material generated by a stable element is called the standard molar enthalpy of the pure. ΔrHθm(298.15K)=∑vBΔfHθm(B, 298.15K)
ΔcHθm: In the standard state (100Kpa, 298.15K), the standard molar enthalpy of the substance completely oxidized to a specified product at the same temperature is called the standard molar combustion enthalpy of the substance. ΔrHθm(298.15K)= -∑vBΔcHθm(B, 298.15K)
The second law of thermodynamics
Entropy (S): Entropy is a state function that reflects the degree of confusion in the motion of the particles. The greater the system chaos, the greater the entropy value. The entropy of any pure perfect crystal at absolute zero is zero. Criterion: ΔS≥0, = 0 reversible, >0 irreversible.
Scope of application: isolation system or thermal insulation system.
Helmholtz free energy (A): During isothermal processes, the maximum work that a closed system can do is equal to the reduction of its Helmholtz free energy. Criterion: A=U-TS ΔA≤0, scope of application: isothermal, isometer does not do other work.
Gibbs Gibbs (G): Under isothermal, isobaric conditions, the maximum non-expansion work that a closed system can do is equal to the reduction of its Gibbs free energy. Criterion: G=H-TS ΔG≤0, scope of application: isothermal, isobaric do not do other work.
Multi-component systems
Partial molarity: In a uniform multi-component system, a certain capacity property of the system is not equal to the sum of the capacity properties of each pure component, but the partial molarity of the broadness of each component and the product of the amount of its substance are additive.
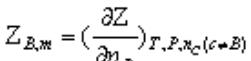
edit
Add a picture annotation of up to 140 words (optional)
。 The physical significance is to keep the number of components other than B unchanged under isothermal and isobaric conditions, and the change in the capacity property Z of the system caused by the addition of 1molB.
Summation formula: The total volume of the V=n1V1+n2V2 system is equal to the sum of the products of the partial molar volumes of each component, V1 and V2, and the amount of their species.
Phase equilibrium
Phase law: f + Φ = (S-R-R') + 2 let C = S-R-R', C is called the group fraction, the expression is f + Φ = C + 2
Two-phase equilibrium of a one-component system: C=1, f=2 at Φ=1; Φ=2, then f=1.
Phase diagrams of two-component systems and their applications: C=2, f=4-Φ, at most 3 degrees of freedom. Note that the p-x plot and the T-x plot have a maximum degree of freedom of 2 and a maximum of 3 coexisting phases.
The positive deviation is very large, the higher the vapor pressure, the lower the boiling point, the highest point on the p-x graph, the lowest point on the T-x graph, and this lowest point is the lowest constant boiling point. If the negative deviation is large, then the opposite is true.
n (1)NH4Cl(s)==NH3(g)+HCl(g) C= S-R-R’=3-1-1=1 Φ=2 f= C+2-Φ=1
(2) C= S-R-R’=3-1-0=2 Φ=2 f= C+2-Φ=2 (3) NH4HS(s)==NH3(g)+H2S(g) 结果同上 (4) C(s)+1/2O2(g)==CO(g);CO(g)+1/2O2(g)==CO2(g); C(s)+O2(g)==CO2(g); C(s)+CO2(g)==2CO(g) 实际存在两个独立化学平衡式C= S-R-R’=4-2-0=2 Φ=2 f(900K)=C+1-Φ=1
n (1)OA is the curve of the crystal shape transformation temperature and pressure change of diamond and graphite, and the two phases coexist in balance on the line. OB is the curve of graphite's melting point changing with pressure, and the two phases of graphite and liquid carbon coexist in balance on the line. OC is the curve of the melting point of diamond with pressure, and the two phases of diamond and liquid carbon are balanced on the line. (2) The O point is the three-phase equilibrium point of diamond graphite and liquid carbon, and the degrees of freedom f=0 (3) At room temperature and pressure, graphite is more stable. (4) At 2000K, the molar volume of graphite is greater than the molar volume of diamond (5) as can be seen from the figure, and a pressure of more than 5 * 10 ^ 9Pa is required.
Edit toggle to center
(3)
Electrolyte solution
The electromotive force of a reversible battery
4. What are the common symbols when expressing a battery in writing? Why is the electrode potential positive and negative? Can I measure a negative electromotive force with experiments?
Chemical kinetics
Reaction rate: The rate at which the progress of the reaction changes over time
Reactants take -, products take +
Primitive reactions: Reactions that are completed in one step
Rate equation: Represents the relationship between parameters such as rate and concentration
K is the rate constant; series = a + b
Zero-level reflection characteristics: concentration * time -1, CA=CAo-kt, t1/2=CAo/2k
Characteristics of the primary reaction: time-1, InCA=InCAo-kt, where InCA-t is linear, and t1/2=In2/k=0.693/k
Secondary reaction characteristics: concentration -1 time-1, 1/CA-t straight line, t1/2=1/KCAo
Surface Physical Chemistry
Surface tension: Molecules in liquids tend to shrink the surface to a minimum. F=2γl (γ is the surface tension)
γ can also be called specific surface Gibbs free energy, which refers to the increase in the free energy of Gibbs for each increase in unit surface area.
Inorganic salts, non-volatile acids and bases, etc. increase surface tension, and solutes that can reduce the surface tension of water are organic compounds.
Additional pressure: When balanced, the force acting on the boundary will have a combined force, and when the liquid level is convex, it will point to the inside of the liquid, and vice versa, the outside. The direction of the additional pressure is the center of the circle pointing to the surface. The smaller the surface area, the more stable the ball
vapour pressure:
This indicates that the smaller the droplet, the greater the vapor pressure.
Surface adsorption: The concentration of a film on a surface is always different from the inside. Both the shrinking surface area and the reduced tension reduce the free energy.