According to the news on the website of the State Food and Drug Administration on January 11, the State Drug Administration issued an announcement and decided to stop the production, sales and use of Lianbizhi injection in China from now on, and cancel the drug registration certificate. The products that have been put on the market shall be recalled by the holder of the drug marketing authorization, and the recalled products shall be supervised and destroyed by the provincial drug supervision and management department of the place where they are located or other harmless treatment shall be taken in accordance with the law.
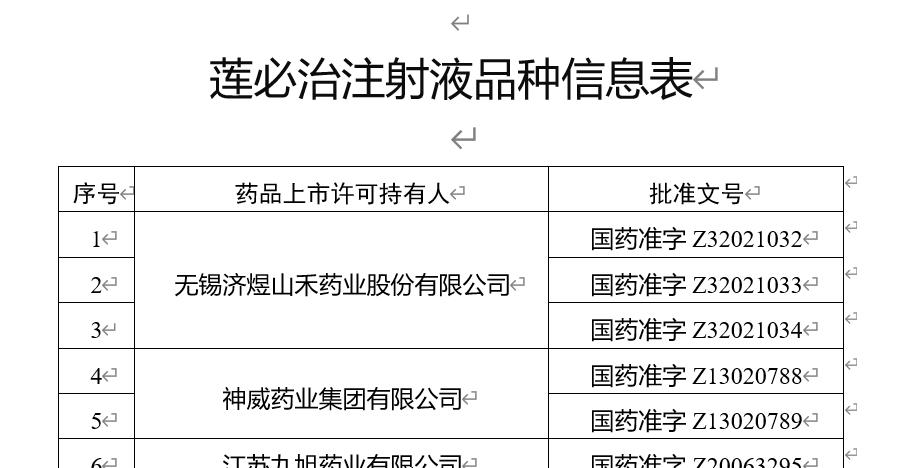
From the variety information table released by the Food and Drug Administration, it can be seen that the variety involves 6 approval numbers of Wuxi Jiyushanhe Pharmaceutical Co., Ltd., Shenwei Pharmaceutical Group Co., Ltd., and Jiangsu Jiuxu Pharmaceutical Co., Ltd. Among them, Shenwei Pharmaceutical (02877. HK) is a Hong Kong listed company.
According to Tianyancha information, Wuxi JiyuShanhe Pharmaceutical Co., Ltd. is 80% controlled by Jiangxi Jimin Trust group co., Ltd., and Jiangsu Jiuxu Pharmaceutical Co., Ltd. is 59% owned by Jiuxu Pharmaceutical Group Co., Ltd.
According to the public information on the official website of the State Food and Drug Administration, the composition of Lianbizhi injection is sodium bisulfite andrographolide, which has the effect of clearing heat, detoxification, antibacterial and anti-inflammatory, and is clinically used for the treatment of bacterial dysentery, pneumonia and acute tonsillitis. The route of administration is intramuscular and intravenous infusion.
In April 2005, the State Food and Drug Administration reported Lianbizhi injection and acute renal function damage in the Adverse Drug Reaction Information Circular (No. 8). In November 2006, the State Food and Drug Administration issued a notice on revising the instructions for Lianbizhi injection, requiring adverse reactions, contraindications, precautions and other items of the drug, of which the adverse reaction requirements were revised to "The existing data suggests that this product may cause rashes, dizziness, gastrointestinal reactions, anaphylactoid reactions, etc., and a small number of patients may have acute kidney function damage." ”